Table of Contents
In chimney or furnace gases the estimations usually made are the percentages by volume of oxygen, nitrogen, carbon-monoxide, and carbon-dioxide. The more refined methods of gas analysis are too slow for technical requirements, and are replaced by the Elliott, Hempel, or other methods. The method here to be described is that of Hempel, and differs chiefly from the Elliott and others in the design of the apparatus, the procedure in each case being the removal of constituent after constituent by certain absorbents.
Apparatus
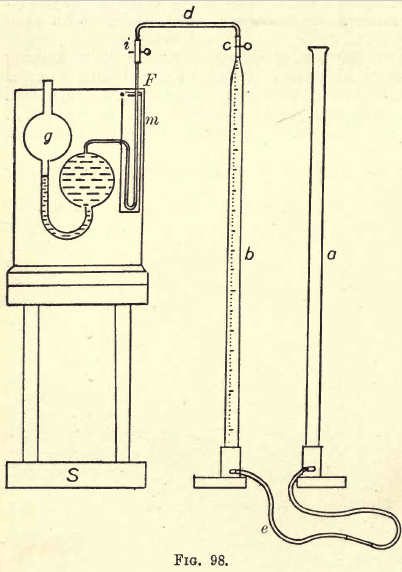
Hempel’s Gas Burette. — The gas burette, as shown in fig. 98, consists of two parts—the levelling tube, a, and the calibrated tube, b. This tube b is of uniform diameter, and ends above in a capillary tube about .5 mm. in diameter and 3 cm. long; and at the other end the tube tapers to
about one-third of its previous diameter. It is here bent at an angle and passes through the wooden up-right on the base. (For the modified Winkler Gas Burette, see Hempel’s Gas Analysis.) The tube b is graduated from the top down in 1/5 c.cs. Total graduation 100 c.cs. The lower end of the tube b is connected by stout rubber tubing (4 feet) to the lower end of tube a, which is of even bore and ungraduated, and fastened as before to an upright oil an iron base. On the capillary tube, c, a short piece of rubber tubing (thick) is wired on, and clamped with a strong Mohr pinchcock. At d a piece of capillary glass tube is connected between the tube and pipettes. A wooden stand, S, is used in conjunction with the burette and pipettes. One pipette must be used for each absorption. For the gases considered three pipettes are required. The simple absorption pipette is shown in place in the figure, and consists of two glass bulbs, connected with a glass tube, bent as shown, and at one end is a capillary tube and white plate; at the other end a wide tube, through which the pipette is filled and emptied. When not in use these ends are closed, one by a cock, and the other by a rubber tube and clip (or glass rod). The whole is mounted on a stand, affixed to which is a label indicating the nature of the absorbent. In the pipette the gas is agitated with the absorbent. This simple pipette will be used to absorb CO2 by KHO. For the absorption of O and CO the compound pipette is used, as shown in fig. 99, as the reagents are acted on by the air.
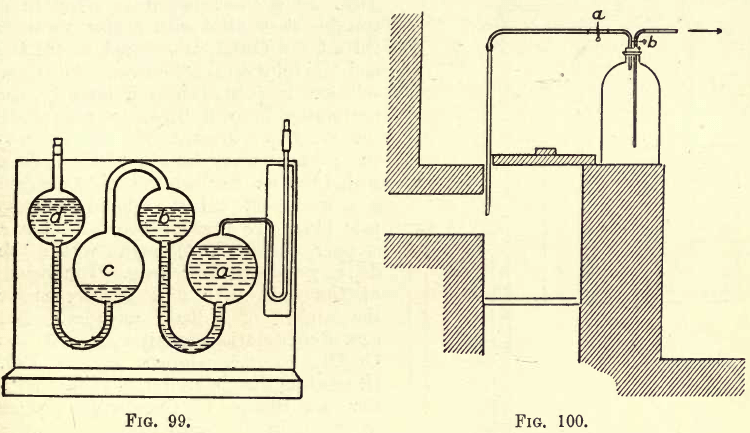
When absorbing O by pyrogallol, a is filled with the pyrogallol solution (see p. 282), b is partly filled with the same solution, c is nearly empty, and d contains distilled water.
The manipulation of the Burette and Pipettes.—The necessary determinations are as follows :—
(a) Carbon dioxide.
(b) Oxygen.
(c) Carbon monoxide.
(d) Nitrogen by difference.
(a) Carbon Dioxide.—If a furnace gas, take the sample just where the visible flame ends. In the case of an assay furnace, introduce an iron tube through a hole in the flue or through a crevice between the lid and
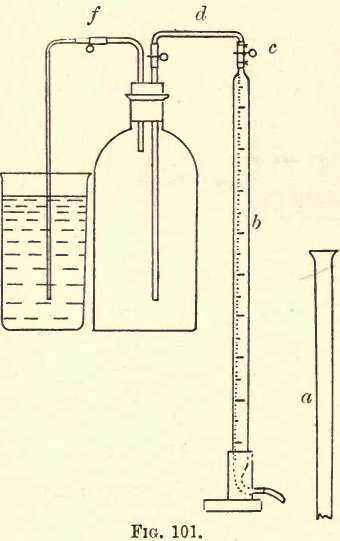
furnace. To the outer end connect a lead or rubber pipe. Take a clean ‘‘Winchester quart” and fit it with a cork and tubes as in fig. 100.
Apply suction by an exhaust syringe till all the air is replaced by the furnace gas (for fuller details consult “ Hempel ”). Clamp the rubber connections at a and b and remove the Winchester and fittings. Having procured a sample of the gas, proceed to fill the three pipettes. Make a 33% solution of KHO (1 to 2). Do not use the brand “purified by alcohol.” 1 c.c. of this solution can absorb 40 c.cs. CO2. Transfer to
pipette as in fig. 98. (The “solid pipette” with rolls of iron gauze is recommended by Hempel.)
For the “ oxygen pipette ” prepare the solution as follows:—Dissolve 5 gms. pyrogallic acid in 15 cms. water and 120 gms. KHO dissolved in 80 c.cs. H2O. KHO by alcohol not to be used.) Charge the pipette as shown in fig. 99. The pipette must be so filled that when the absorbent in a is forced up and fills h, the distilled water in c is forced up into and fills d. Invert the pipette, dip the capillary in the absorbent, and fill a by suction. Drive the absorbent into b and fill d with water. Then by suction bring back the absorbent to the capillary.
For the “carbon monoxide pipette” dissolve 10.3 gms. copper oxide in 100 to 200 c.cs. of concentrated HCl. Then allow the solution to stand in a suitable flask filled with copper turnings till all the CuCl2 is reduced to Cu2Cl2 and the solution is colourless. The clear solution is poured into a large beaker containing 1 to 2 litres to precipitate the Cu2Cl2. Decant the dilute solution ; then wash into a 250 c.c. flask with 150 c.cs. distilled water. Ammonia gas is passed till the liquid becomes pale blue. To prevent access of air a stopper is used, through which the delivery and exit tubes pass. The mouth of the exit tube dips slightly under the surface of a little mercury. 100 c.cs. of the solution contain about 7.3 gms.
Cu2Cl2. 1 c.c. absorbs 6 c.cs. CO. (Regarding the deterioration of this solution, see Hempel.) Charge the pipette as shown in the figure (99).
Thoroughly clean the gas burette by washing. Check by weighing (or measuring) the calibration of the burette. Fill the tubes a and b with water, raising and lowering one of the tubes till all air bubbles are removed.
Join the burette to the Winchester by a glass tube filled with water by raising the tube a. Dip the bent arm of the Winchester in a large beakerful of water (fig. 101). Open the clamps on the Winchester. Take
the tube a in the left hand, and firmly close the tube at e (fig. 98) by gripping between the finger and the palm of the hand. Pour out the water in a.
Place a on the table and open the clamp c. The gas is now drawn into the burette. Draw in a little more than 100 c.cs. Close the clamp c. Disconnect the Winchester. Let the water run down the sides. Now compress the gas to less than 100 c.cs. by raising a. Grip the rubber tube at e. Set a on the table. Raise b to the level of the eyes. Slightly release the grip and allow the water to run back to 100, gripping firmly. Open c for a moment and the excess of gas escapes, and there remain in the burette 100 c.cs. gas under atmospheric pressure.
The absorption of the CO2.—Set the KHO pipette on the stand. The bulb g is empty. Connect the pipette to c as shown in fig. 98, thus: fill the little tube d with water; then introduce capillary F which is thus filled with water. The tube i is squeezed between the fingers to expel the air, and P is immediately inserted. Raise a and the gas passes into the pipette. With care the bubble of air introduced in the capillary should not be longer than 10 mms. When all the gas has passed into the pipette, allow about ½ c.c. of water to follow. Clamp e. Remove the pipette, clamp i, and shake for about three minutes. Reconnect. Lower a. Unclamp c and i and run back the gas, taking care that the KHO does not pass further than F. Clamp e. A better practice is to make a fixed mark at m, place a clamp at i, and always fill the tube F as well as the burette b with the gas. All measurements are then made with the absorbent at the mark m. Read the volume of the remaining gas by moving a till the water level is the same in both tubes. Note the reading.
Absorption of the Oxygen
The burette is now connected with the double pipette containing the pyrogallol solution. The absorption takes about 5 minutes, and must be performed by repeated agitation. The gas remaining is returned to the burette and measured as before. Note the
result.
Absorption of Carbon Monoxide
The burette is now placed in communication with the CO compound pipette. The absorption here is somewhat slow, and requires frequent agitation for a period of 10 to 15 minutes. In this process a certain amount of gaseous HCl may become mixed with the gas. Transfer the gas to the burette; then for a few minutes to the KHO pipette; then back to the burette. Read as before, and note the result.
(d) Nitrogen by Difference.—In the mixture considered the nitrogen now remains, and its volume is obtained by the last reading of the burette.
The following figures are given to show the method of calculation:—

When finished with each pipette, the student should note on a small label the number of c.cs. of gas absorbed. Each pipette may be used a number of times, and this practice serves as a guide when the absorbing power of the solution is in question.
When the student has finished this estimation he should proceed with another of the same sample till concordant results are obtained. It will be sufficient for the purpose of this text-book if the student master the manipulation of the simple Hempel burette and pipettes. He is referred to the School of Mines Quarterly for a full description of the Elliott apparatus, which is somewhat simpler than the Hempel, and to Hempel’s Gas Analysis for modifications of the simple burette and for an elaborate examination into the accuracy of the technical versus the more refined methods of gas analysis. It is sufficient to state here that unless special accuracy be required for some very particular case, the methods mentioned yield very satisfactory results.
