Table of Contents
In this section eight chapters are given, each dealing with one or more technical methods of analysis. The typical technical method should be quick, accurate, and inexpensive. Accuracy may to some extent be sacrificed in favour of speed when the quantity and effect of such inaccuracy are approximately known, yet it should always be the aim of the technical chemist to combine accuracy and speed. The past twenty years have seen great advances in technical methods, but with all the advance made it must be admitted that much still remains to be done. New methods of analysis are almost daily being brought forward and published in the current chemical and metallurgical literature. Of these methods—like parents— only a few stand the test of practice. Too often it is found on trial that their range of application is too limited, or that their claims to accuracy and speed are doubtful. This being so, the student must in his future professional work receive with caution any new method applying particularly to his special work. It is his duty to test the method from the points of view of accuracy, speed, and range of application. Under this latter point he must test the effect of the presence of various impurities on the accuracy of the results. If these results are satisfactory, he may employ the method in his daily work.
Of the methods given in the following pages it will be seen that some approach the ideal in accuracy and speed, but many of the others, though accurate, fail in speed.
Introductory
The analysis of water varies in its methods according to the end in view, i.e. whether the water is to be used for drinking purposes, or for the generation of steam or other purposes in the arts. The analysis for drinking purposes, termed Sanitary Analysis, will not be considered here (see Sutton’s Volumetric Analysis). When considering the suitability of a water for boiler use, the objectionable impurities may either be in suspension or in solution. In the former case they may be removed by filtration, in the latter other means must be adopted. The chemist then has to estimate the quantity and nature of the impurities present, and often is expected to advise regarding the treatment of the water. On this latter point the student may consult such works as Phillips or Stillman on engineering chemistry. The following tabulation shows the chief impurities met with.
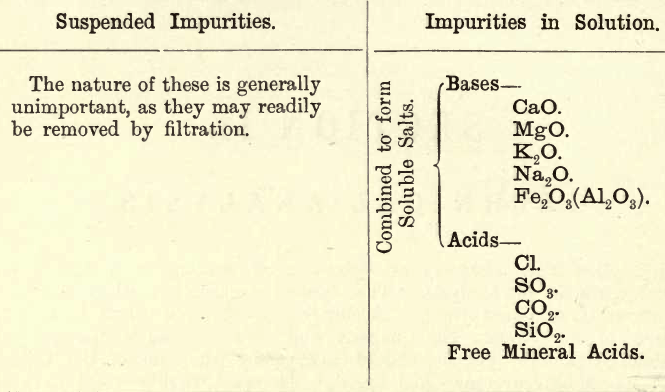
In addition to these it is sometimes necessary that the amount of oxygen in solution be determined.
Generally the Cl is combined as follows—first with Na, then with K, then with Mg, then with Ca. The sulphuric acid combines with the alkalies Na and K provided there is not enough Cl to saturate them, then with Ca, then with Mg. The carbon dioxide, after the above combinations have been made, unites with the Ca and then with the Mg. Certain mineral and artesian well waters form exceptions to this general rule.
In practice certain of these impurities form scale in the boilers. This scale generally consists of carbonates and hydrates of lime and magnesia; sulphate of lime, with some iron alumina, combined water, etc.
Other impurities have a corrosive action. Therefore, to be of service to the mechanical engineer, it is advisable to classify waters thus:—
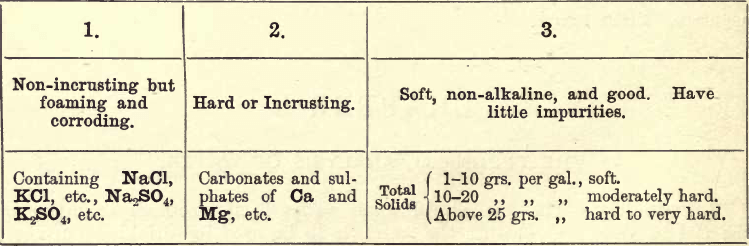
Of Class 1, MgCl and MgSO4 are very obnoxious. Ten grains per gallon of these impurities should condemn the water for boiler use, as in contact they are easily decomposed by iron, and rapidly corrode it.
Of Class 2, up to about 40 grs. per gallon can be dealt with by ‘boiler compounds,’ consisting of alkaline carbonates, but above this the added alkali is apt to cause foaming.
Of Class 3, little need be said. The figures given are somewhat relative, and what is considered a hard water in one district may be much preferable to what is considered soft in another. The figures given form an approximate standard (see Stillman). The student bearing the above points in view will now be in a position to intelligently investigate a sample of boiler water.
Method of Analysis
An excellent rapid method is that of Prof. Wm. Main (see Stillman).
In the method here given the alkalies are determined directly, and not by difference as in Main’s method. Briefly, then, the necessary estimations are as follows:—
(а) By evaporation, estimate the total solids,
(b) By ignition estimate the organic and volatile matter.
(c) Treat the residue with HCl; the greater part is dissolved. In this estimate CaO, MgO, Fe2O3, Al2O3, and the alkalies (before precipitating MgO, or in a fresh sample).
(d) In the residue that still remains from (c) estimate by fusion the insoluble SiO2, Fe2O3, Al2O3, and CaSO4.
(e) The CO2 may be determined directly in a fresh sample or by calculation.
(f) The Cl is determined, as usual, in a fresh sample.
(g) The SO3 is determined in a fresh sample.
Estimation of Total Solids
(Suspended matter is first removed and estimated by filtration.)—Take 700 c.cs. of an average sample of the water in question, and transfer till two-thirds full to a weighed platinum basin (a porcelain dish will do in case of emergency, but is not so satisfactory). Place the dish and contents on an asbestos board, on a tripod, over a rose bunsen burner. Adjust the flame so that the water is nearly but not quite boiling. Continue the evaporation, adding when necessary the remainder of the 700 c.cs. When just dry transfer to an air bath, and heat at 110° C. till the weight is constant. Enter all weights in the note-book.
Deduct the weight of the platinum dish from that of the dish + residue.
The difference is the weight of the total solids. The result is generally expressed in ‘ grains per gallon.’ The United States gallon contains 231 cubic inches, and the British imperial gallon 277.274 cubic inches. Now, as 700 c.cs. of water were taken, the student will find by calculation on the basis of 1 gal. = 10 lbs. = 70,000 grs. that the weight of the residue in centigrams gives grains per gallon. Thus, if the residue weigh .0412 gm., the total solids are 4.12 grains per gallon. If the results are required as so many parts per million, multiply grains per gallon by 14.285. These results are based on the British standard, and, with the required proportional alteration, are easily adapted to the United States standard.
Organic and Volatile Matter
Take now the dish and its contents and heat at a dull red till no more fumes seem to be given off and the residue is white or nearly so. Besides organic matter, CO2 will have been driven off by this ignition. This CO2 is now replaced as follows:—Add about 50 c.cs. of water saturated with CO2, and evaporate nearly to dryness; again add 50 c.cs. of the saturated water and evaporate carefully to dryness. When just dry transfer to the air bath at 110° C., and treat as before till constant.
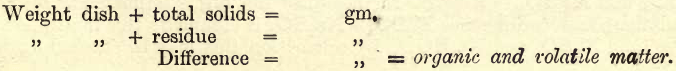
Calculate as before to grains per gallon. Any inaccuracy that may arise through imperfect replacement of the CO2 may be checked in the final combination of results.
Analysis of the Residue
For CaO, MgO, Fe2O3, Al2O3, SiO2, and Alkalies.—Moisten the residue in the dish with a few drops of strong HCl. Add 50 c.cs. dil HCl (E.). Evaporate to dryness, and heat at 110° C. till there is no more odour of acid fumes. Add 50 c.cs. hot water and a few drops of strong HCl. Transfer dish and contents (see that the outside of the dish is perfectly clean) to a beaker and boil for a few minutes. Filter through a small filter (5 cm.). Wash well with hot water (till no reaction is obtained for HCl). Reserve the filtrate.
Dry the filter and contents, ignite and weigh. Report as grains per gallon ‘insoluble residue,’ chiefly SiO2, with a little Al2O3, and perhaps traces of Fe2O3 and CaSO4. For most purposes it will be sufficient to report this as insoluble siliceous residue. If, however, further accuracy is required, fuse this residue with carbonates of Na and K in a platinum crucible, and estimate SiO2 contents; then, if necessary, the Fe2O3: Al2O3, CaO, etc. may be estimated. The student may with advantage omit at present this examination of the insoluble residue, and consider the results as ‘ siliceous matter ’ in grains per gallon.
Whilst the drying and ignition of the insoluble residue is proceeding, the filtrate is treated as follows:—Add 2 drops strong HNO3; boil for a few minutes to oxidise ferrous compounds. Cool slightly, and make slightly alkaline with NH4HO. Hydrates of Fe and Al are precipitated. Boil for 5 minutes to expel part of the excess of NH4HO. Filter, reserving filtrate (preferably through a Gooch crucible); ignite and weigh. The difference between the weight of the crucible and that of the crucible and contents represents Fe2O3 + Al2O3. Calculate as usual to ‘alumina and iron,’ grains per gallon.
The filtrate is now made decidedly alkaline with NH4HO, and an excess of E. (NH4)2C2O4 is added. Boil for 3 or 4 minutes. Filter, reserving filtrate (preferably Gooch). Wash well with hot water (test). If much MgO is present, the precaution before mentioned of redissolving and reprecipitating must be observed. Ignite the precipitate first over the bunsen and then over the blast till constant CaC2O4 = CaO + CO + CO2. Calculate the result as grains CaO per gallon.
The filtrate from the lime is evaporated to about 100 c.cs. and excess of E. Na2HPO4 solution. Stir rapidly with a light glass rod for a few minutes, avoiding touching the sides. Set aside in a cool place for three hours.
Note.—To save time, the student may now proceed with the estimation of Cl, SO3, and alkalies.
Filter through a small filter and wash well with a dilute solution of NH4NO3. (the “Gooch” is preferable, the NH4NO3 then being unnecessary.) Dry, ignite (the NH4NO3 in the pores of the paper aids ignition), and weigh. The ignited residue should consist of Mg2P2O7, which contains 36.024 per cent. MgO. Multiply the weight of the precipitate by .36024 and calculate as before, the result being grains MgO per gallon.
In the duplicate estimation the student may estimate the alkalies in the filtrate from the lime. This filtrate is divided into two equal portions (measured). One is evaporated to dryness with a few c.cs. dilute H2SO4, forming K2SO4, Na2SO4, and MgSO4. Weigh. In the other the Mg is estimated as MgSO4 (calculation), and by difference K2SO4 + Na2SO4 is obtained ; this multiplied by 2 gives total K2SO4 and Na2SO4. By calculation this may be checked against the results obtained by the following method :— Take a fresh sample of the water, say 700 c.cs. (see above note re saving of time), and evaporate in a platinum dish to about 100 c.c. Add a few drops strong HCl and then Ba(OH)2 solution till strongly alkaline. Boil, filter, washing well with hot water till free from Cl. To the filtrate add (NH4)2CO3 till a precipitate ceases forming. Boil and filter off the p’p’t. Wash well and evaporate the filtrate to dryness, and heat at a very dull red to drive off NH4Cl. This treatment removes the MgO more or less completely ; but as some may still be retained by the alkaline chlorides, the residue is dissolved in a small quantity of hot water, and the treatment with Ba(OH)2 and (NH4)2CO3 repeated, the resulting solution being evaporated to dryness, and the NH4Cl expelled at a very dull red heat. When the weight of dish and contents is constant, the result is entered and calculated to grains NaCl + KCl per gallon. If required, the NaCl and KCl may be separated as described in the chapter on the Analysis of Silicates. (For the present the student may, if his time is too limited, omit this estimation.)
In the early part of the analysis, when a residue was obtained by evaporating 700 c.cs. water to dryness, the weight of this residue was noted after incineration, and replacement of any CO2 thus expelled. This residue (in a fresh sample) may be analysed for CO2 and combined H2O as before described. This estimation may, however, be omitted in a technical analysis, unless for some particular reason it be desirable that the CO2 be determined.
Chlorine may be quickly aud accurately estimated volumetrically by a standard solution of AgNO3, with K2CrO4 as an indicator.
Take 70 c.cs. of the water in a 100 c.c. porcelain dish, add two drops K2CrO4 (E.) solution. Titrate from a burette with the standard silver solution (4.79 gm. pure AgNO3 per litre), stirring till the colour just changes from yellow to yellowish red. The silver has a strong affinity for chlorine, and silver chromate (red) is not formed permanently till all the chlorine is combined with silver. Any that is momentarily formed is broken up thus—
Ag2CrO4 + NaCl = Na2CrO4 + 2AgCl
Note the number of c.cs. used, and deduct .1 c.c. absorbed in colouring the solution. Multiply the number of c.cs. by .001 and then by 10 (briefly, by .01), and read the result in grains chlorine per gallon. Take 700 c.cs. of the water. Acidulate with 10 c.cs. HCl (5E.), and evaporate to about 200 c.cs. in a large porcelain dish. Transfer to a beaker. Heat to boiling, and add, with constant stirring, 10 c.cs. BaCl2 (E.) solution. Set aside for twelve hours. Filter through a Gooch, dry, ignite, and weigh the Ba2SO4, and estimate the SO3 in grains per gallon.
Combination of Results.—These are now, for example, as follows :—
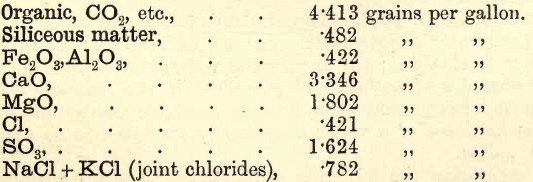
Note.—The Cl in this NaCl and KCl may be partly introduced in analysis.
The student must now combine these results according to the general rules previously laid down. The siliceous matter and Fe2O3,Al2O3 remain as they are. From the total solids a fair idea of the nature of the water is obtained. These in the example quoted are low ; the sample may be regarded as good. As the Na2O and K2O were not separately estimated, the following example from Stillman may be taken and worked out by the student:—
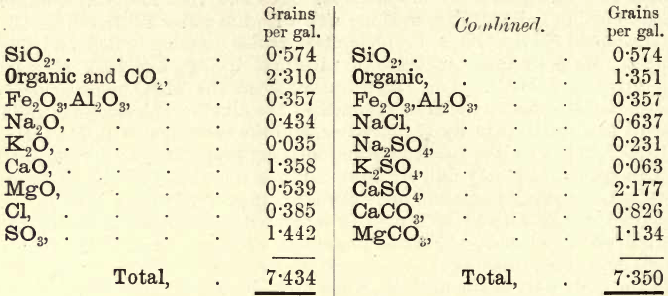
The Cl is first united with Na2O. Any balance of Na2O is united with SO3. (If the balance consisted of Cl, it is then united with K2O.) The K2O is now combined with SO3. If any SO3 remains it is combined with CaO ; and if SO3 still remains, then combine it with MgO ; but if not, the balance of CaO is united with CO2, and the MgO is united with CO2, the total CO2 thus absorbed being deducted from the item.
Organic + CO2.—On working through this calculation, the student will notice the continuity of the method. Unless the Na2O and K2O are estimated, only approximate results are obtained. In many cases, however, these results are sufficient; but where the proportion of chlorine is more than a few grains per gallon, the alkalies must be fully determined.
Evaluate Hardness of Water
Water containing certain salts of lime, magnesia, etc. in solution is said to be hard. Certain of the salts, notably the carbonates of calcium and magnesium, can be almost entirely precipitated by boiling the water. The hardness due to these carbonates is termed ‘ temporary hardness ’; that due to compounds such as calcium sulphate, magnesium chloride, etc., which are not precipitated on boiling under atmospheric pressure, is termed ‘ permanent hardness.’
The temporary and permanent hardness may be determined by titration with standard H2SO4, which gives the temporary hardness, and followed by precipitation on boiling with Na2CO3 (a known volume), and titration of the filtrate with H2SO4 for excess of Na2CO3. The following method is given by most writers on this subject.
Dr Clark’S Soap Test
The student will notice that on washing his hands in a hard water a considerable amount of soap is consumed before a permanent lather is obtained; the harder the water, the more soap is consumed. The soluble stearates of sodium and potassium in the soap are decomposed by the calcium and magnesium salts, forming insoluble compounds which give no lather. The hardness of a water is generally expressed as so many grains of CaCO3 per gallon.
In this estimation the following standard solutions are required—standard hard water and standard soap solutions.
Standard Hard Water
Dissolve 1.11 gms. pure CaCl2 in a little distilled water, and dilute with distilled water to 1000 c.cs. (measured at 15° C.). One c.c. of this solution will correspond with .001 gm. CaCO3.
Standard Soap Solution
Castile soap may be used for this purpose, but in cold weather Phillips recommends the use of sodium oleate, about 13 gms. being dissolved in a mixture of 500 c.cs. methylated spirit and 500 c.cs. distilled water, filtering if necessary. To standardise this soap solution run in 12 c.cs. hard water standard solution into a 250 c.c. stoppered bottle. Fill a burette with the soap solution and run in 1 c.c. at a time, vigorously shaking after each addition, till a point is reached where a permanent lather is obtained. Note the number of c.cs. 12 c.cs. of the water should take 13 c.cs. soap solution, as 1 c.c. soap solution is required to make a permanent lather with distilled water alone. Suppose, however, that only 11.5 c.cs. soap solution were required. Then to every 11.5 c.cs. soap solution 13- 11.5 = 1.5 c.cs. of water must be added. Dilute accordingly with water and spirits. Check again on the standard hard water, and re-correct if necessary.
Total Hardness
Take 70 c.cs. of the water under examination and transfer to a 250 c.c. stoppered bottle. Titrate with standard soap till a permanent lather is obtained. Note the number of c.cs. used. Deduct 1 c.c. (necessary to bring about the reaction with distilled water). The number of c.cs. gives the hardness in degrees Clark or in grains CaCO3 per gallon (equivalent). If the water takes more than 16 c.cs. soap solution, add 70 c.cs. distilled water, and proceed as before. The lather will thus form more uniformly. When the water contains a fair percentage of magnesia salts the lather forms slowly. A little experience and experiment are necessary when dealing with such waters.
Permanent Hardness
Boil 250 c.cs. of the water for one hour, keeping up to about the same volume by additions of distilled water (boiling to expel CO2). Cool. Make up to 250 c.c. Mix well, and pour out 70 c.cs. into a bottle, and titrate as before. The permanent hardness is thus ascertained.
The Temporary Hardness is obtained by deducting the permanent from the total hardness.
For methods of estimating the oxygen in water consult Sutton’s Volumetric Analysis.
We have recently put together a report on what we think are the best whole home water filters in 2022.
