Table of Contents
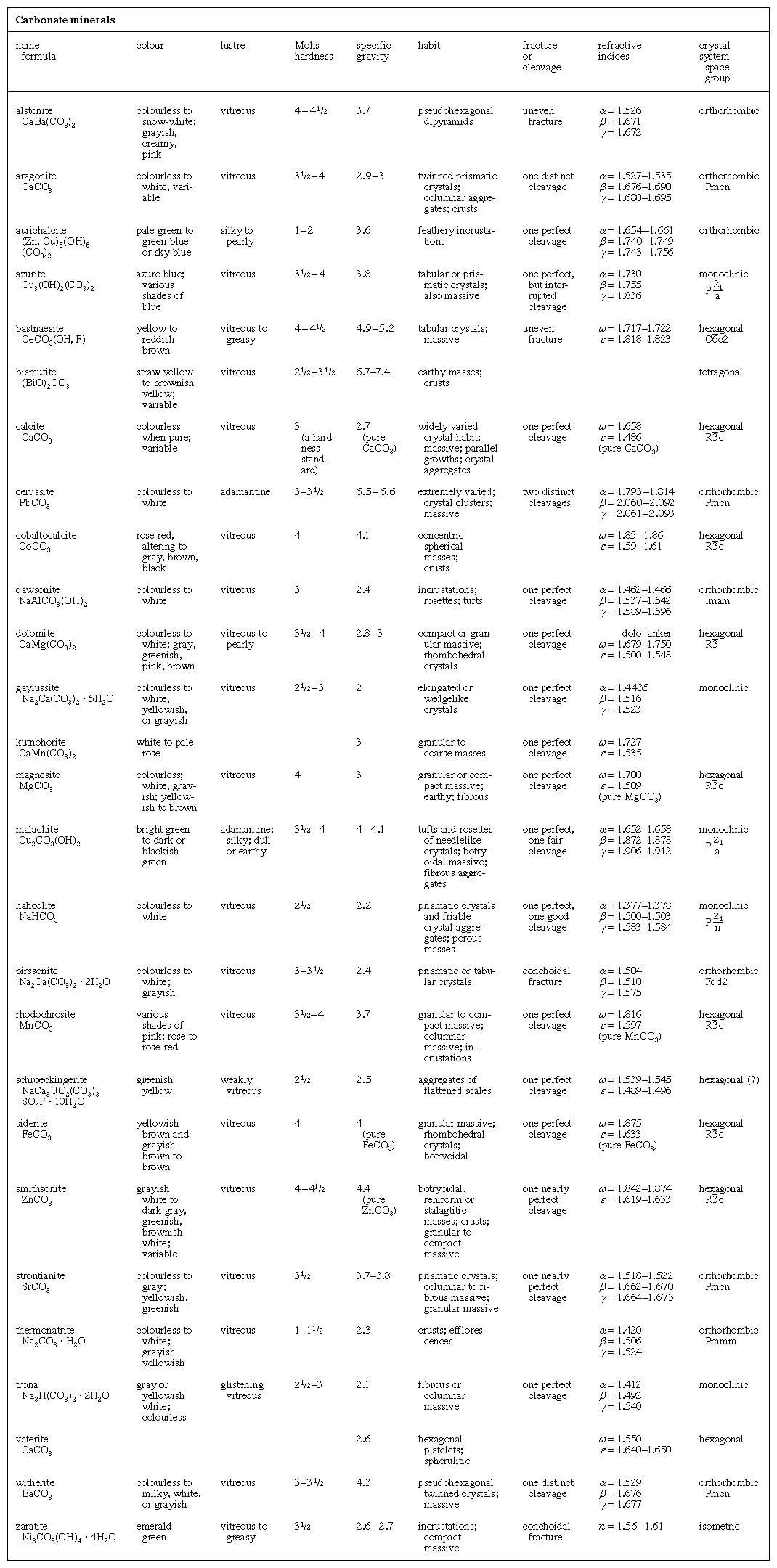
This is an introduction list that covers the most common carbonates minerals which are compounds of carbon dioxide (frequently called carbonic acid) with oxides of metals. In naming individual carbonates, the name of the metal is used; but if the name of the oxide of the metal is better known, as lime, for example, which is calcium oxide, the name of the oxide will be used instead of the name of the metal in the following descriptions. Carbonates can be “spotted” by testing with acids, which cause “fizzing,” owing to the escape of carbon dioxide. All carbonates are non-metallic in luster.
Calcite or Calcspar
Calcite or Calcspar; CaC03. — Color, white or colorless, also pale shades of gray, red, green, blue, violet, yellow, and sometimes brown and black, from impurities; powder, white or grayish; luster, glassy or earthy; H = 3; G = 2.714; crystals, in hexagonal system; cleavage, very perfect, cleavage pieces having angles of about 75° and 105° over the edges. 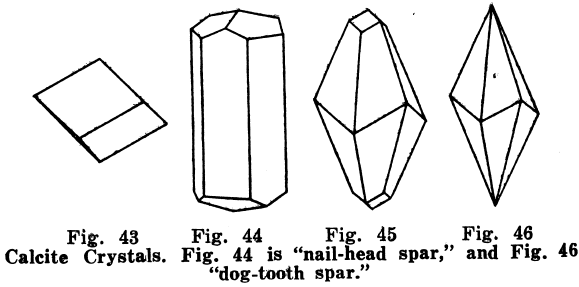
The word or syllable, spar, in the names of minerals refers to bright surfaces, particularly when due to noticeably good cleavage. Calcite is carbonate of lime, and contains 44% carbon dioxide and 56% of lime; it effervesces (fizzes) with acids, even when the acids are mixed with water, i.e., diluted. Vinegar may be used for this test, and no heating is required.
Varieties of Calcite. — Dog-tooth spar, sharp-pointed crystals (Fig. 46) Nailhead spar, blunt-pointed crystals, (Fig. 44). Iceland spar, very perfect, transparent, colorless crystals. Satin spar, fibrous and of a satiny luster; a variety of gypsum is also called satin spar. Limestone, marble, and chalk are granular or very fine-grained rocks composed of calcite. Lithographic stone is a very fine, even-grained limestone. Hydraulic limestone and calcareous marl are composed of calcite, with more or less clay, etc, Stalactites hanging like icicles from the roofs of caves, and stalagmites, sticking up from the floors, are usually composed of calcite. Oriental alabaster, or onyx marble, and Mexican onyx are calcite formed in layers on the bottoms of caves, or are deposited from springs and rivers, particularly in volcanic regions.
Lithographic stone is a very fine, even-grained limestone. Hydraulic limestone and calcareous marl are composed of calcite, with more or less clay, etc, Stalactites hanging like icicles from the roofs of caves, and stalagmites, sticking up from the floors, are usually composed of calcite. Oriental alabaster, or onyx marble, and Mexican onyx are calcite formed in layers on the bottoms of caves, or are deposited from springs and rivers, particularly in volcanic regions.
The uses of calcite, in the form of limestone, for building stone and marble, for making lime and cement, for fluxing iron ore, etc. are well known. For these purposes, limestone is a cheap material, a dollar or two a ton. Pure calcite is ground to a fine powder, and is used instead of whiting. White calcite of good cleavage is used as stucco dash. Iceland spar (large, very perfect crystals of calcite) is used in microscopes (Nicol’s prism); good pieces, clear, colorless, and free from flaws, are of considerable value ($20 to $40 a pound) but are rarely found free from imperfections.
Dolomite
Dolomite CaCO3 :MgCO3.—Color, red, reddish, or greenish white, sometimes, rose-red, green, brown gray or black; powder, white; luster, glassy; H = 3.5 to 4; G = 2.8 to 2.9; crystals, hexagonal system, like calcite; cleavage, perfect, giving cleavage pieces like those of calcite; composition, carbonate of lime and carbonate of magnesia, in about equal parts. True dolomite fizzes with acid only when heated; but dolomite is often mixed with calcite, which is active with cold acids. Most limestones contain more or less carbonate of magnesia, so that limestones are sometimes dolomitic, and shade into true dolomite. Dolomite is useful as a building stone, and crystalline dolomite forms a part of the marble of commerce. Dolomite is also used in smelting iron ore, the magnesia tending to carry the sulphur into the slag, and thus purifying the pig iron; it is sometimes used instead of common limestone in making the sulphite liquor in wood-pulp manufacture. Value, $2.00 to $4.00 per ton.
Magnesite
Magnesite; MgCO3. — Color, white, yellowish, or grayish white, brown; powder, white; luster, glassy; H = 3.5 to 4.5; G = 3 to 3.12; crystals, in hexagonal system, like calcite; cleavage perfect; magnesite is sometimes found with no appearance of crystals. It then looks much like dull porcelain but is not so hard. Composition, carbonate of magnesia, often mixed with a little carbonate of iron; fizzes with hot acid. Used largely for making magnesia bricks, for lining electric, basic-steel, copper, and other furnaces, and for sulphite-pulp manufacture, fire-proof flooring, etc.; also for manufacturing magnesia medical preparations and various chemicals. Value, $35 to $40 per ton, when dead burned. Found in serpentine, talc schist, and other magnesian rocks, including dolomite.
Hydromagnesite
Hydromagnesite is a carbonate of magnesia, with extra magnesia and water. It is found as tufts of small crystals, and also as chalky crusts.
Siderite or Iron Spar
Siderite, or Iron Spar; FeCO3. — Color, yellowish gray, greenish gray, sometimes brown, or brownish red, or white; powder, white; luster, glassy or pearly; H = 3.5 to 4; G = 3.83 to 3.88; crystals, in hexagonal system, like calcite; cleavage, perfect; often very fine-grained or amorphous; composition, carbonate of iron, containing 48.3% of iron; but it usually contains more or less of the carbonates of manganese, magnesia, and lime, which lower the percentage of iron. Clay ironstone is siderite mixed with clay. Siderite is valuable as an ore of iron, but it usually requires calcination before it can be used.
Ankerite
Ankerite is carbonate of lime, magnesia, and iron. Minerals of various composition between calcite and ankerite are met with; they usually look much like calcite when fresh, but weather to a rusty color (“rusty weathering carbonate”).
As an iron ore, siderite forms extensive deposits in sedimentary rocks. In Ontario, there are very large quantities in the Keewatin iron formation. In Nova Scotia, veins are found of siderite, ankerite, etc., cutting sedimentary rocks near bodies of acid igneous rocks.
Rhodochrosite or Manganese Spar
Rhodochrosite, or Manganese Spar; MnCO3. — Color, rose red, sometimes yellowish gray, fawn colored, dark red, brown; powder, white; luster, glassy or pearly; H = 3.5 to 4.5; G=3.5 to 3.6; crystals, in hexagonal system, like calcite, but not common; composition, manganese carbonate, usually with some iron, lime, and magnesia; fizzes with hot acids. May be used as an ore of manganese. Value, see pyrolusite. Found usually with other ores of manganese; also in veins with ores of gold, silver, lead, and copper.
Strontianite
Strontianite; SrCO3. — Color, green, sometimes white gray, yellowish, brown; powder, white; luster, glassy; H =3.5 to 4; G =3.680 to 3.714; cleavage, good; composition, strontium carbonate; fizzes with acids. This and celestite are commonly called strontium ores, used in making strontium nitrate for red flares and fireworks; also, to make strontium hydrate for use in refining sugar, and for other smaller chemical uses. Value, $100.00 to $200.00 per ton when finely ground. Found mostly in association with lead ores.
Witherite
Witherite; BaCO3, — Color, white, yellowish, grayish; powder, white; luster, glassy, inclining to resinous on broken surfaces; H =3 to 3.75; G=4.27 to 4.35; cleavage, good; composition, barium carbonate; fizzes with acids. When found in quantity, can be used for the same purposes as barite. Used in the manufacture of pressed brick, to neutralize the sulphuric acid formed by oxidation of sulphides; its use prevents the unsightly, white efflorescence. Found mostly with lead ores.
Cerussite or White Lead Ore
Cerussite, or White Lead Ore; PbCO3.— Color, white gray, grayish black, sometimes tinged blue or green with carbonates of copper; powder, white; luster, like diamond or dull, if, very fine-grained; H =3 to 3.5; G = 6.46 to 6.574; very brittle; cleavage, distinct; composition lead carbonate. Cerussite is formed by weathering of galena.
Smithsonite
Smithsonite; ZnCO3.—Also called cala-mine; miners call it dry bone; color, white, often grayish, greenish, brownish white, sometimes green, blue brown; powder white; luster, glassy to pearly; H=5; G=4.30 to 4.45; cleavage perfect; composition, zinc carbonate. Usually found as a product of zinc blende weathering.
Malachite
Malachite; CuCO3·Cu(OH)2. — Color, bright green; powder, pale green; luster, dull and earthy or glassy; H = 3.5 to 4; G = 4; cleavage, perfect; composition, carbonate of copper, etc., containing 57.5% of copper. Formed by weathering of sulphides of copper; sometimes, as at the old Copper Queen, Bisbee, Arizona, there is enough of it to be valuable as a copper ore.
Azurite
Azurite; 2CuCO3·Cu(OH)2. — This is a blue carbonate of copper, carrying 55% of copper, powder, blue; luster, earthy or glassy; H = 3.5 to 4; G = 3.77 to 3.83. Azurite is also a weathering product from sulphides of copper.