Table of Contents
- Magnetite or Magnetic Iron Ore: Fe3O4
- Ilmenite or Titanic Iron Ore: FeTiO3
- Franklinite
- Hematite: Fe2O3
- Limonite: 2F2O33H2O
- Chromite: FeCr2O4
- Tinstone or Cassiterite: SnO2
- Corundum: Al2O3
- Bauxite: Al2O3·2H2O
- Pyrolusite: MnO2
- Zincite or Red Zinc Ore ZnO
- Cuprite or Red Copper Ore: Cu2O
- Rutile (M 82): TiO2
- Quartz: SiO2
Oxide minerals can be listed as compounds of oxygen with metals. A list of the common oxide minerals with the spinel structure, together with their compositions, u values, cell dimension and structure type. Pure end member compositions of these minerals arc rare in nature, and cation substitution, especially between cations of similar size and valence, results in extensive solid solutions between them. An important solid solution is that between magnetite Fe3O4 and Fe2Ti04 as it is the main carrier of magnetism in rocks. One of the questions we will look at in greater detail in a later chapter is the fate of spinel solid solutions crystallised at high temperatures. A solid solution implies random substitution of cations for one another i.e. disorder. We have already seen in general terms that disorder at high temperatures tends to give way to order at lower temperatures. We will come back to this topic after discussing the thermodynamic and kinetic factors which control such behaviour.
Magnetite or Magnetic Iron Ore: Fe3O4
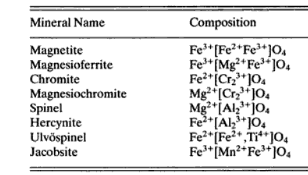
Magnetite and magnetic iron ore has for color, iron-black; powder, black; luster, metallic, but sometimes rather dull; brittle; H = 5.5 to 6.5; G = 5.168 to 5.180; composition (as calculated from the composition formula) iron, 72.4%, oxygen, 27.6%. Sometimes contains titanium, sometimes manganese. Crystals in the cubic system, mostly octahedrons (see Fig. 2), but also dodecahedrons. (Figs. 3 and 4). Cleavage is usually indistinct; but coarse-grained magnetite often breaks with smooth, flat surfaces (parting planes); strongly magnetic. The word magnetic is used in two senses: (a) attracted by a magnet; (b) attracts certain substances (as iron), i.e., it is itself a magnet. Magnetite is always magnetic in the first sense; it is occasionally magnetic in the second sense, in which case, it is called loadstone. (Try its attraction on small pieces of iron.) A piece of loadstone has a north pole and a south pole, like a compass, which property is called polarity. The magnetic property of minerals can be conveniently tested with a magnetized
knife blade; and a blade that has been rubbed with a strong magnet will keep its charge for many years. To test for magnetism, break off a number of small grains of the mineral, and bring the point of the magnetized knife blade to them slowly; if strongly magnetic, the grains will jump to the knife blade. Do not put the point of the blade on the little pieces, as they may stick together by reason of other causes besides magnetism. When testing for hardness by scratching the mineral with the point of the blade, a magnetic mineral will form a fringe around the point. But caution is necessary here; if the mineral is about the same hardness as the blade or harder, the fringe may be made up of bits of steel from the blade.
Magnetite is a common ore. In some localities, as in Sweden, it is valued on account of its purity, being free from phosphorus and sulphur; but, because it is usually harder and less porous than hematite, the latter is preferred, as it is easier to melt. Value, at a port near the furnace; but an ore low in phosphorus and containing 68% of iron is quoted higher.
Taking the specific gravity of the ore as 5, the number of cubic feet per ton is calculated as follows: weight of a cubic foot of water is 62.4 pounds; weight of a cubic foot of magnetite is 62.4 x 5 = 312 pounds; number of cubic feet to the ton is 2000 ÷ 312 = 6.41. Since a cubic yard contains 27 cubic feet, a cubic yard weighs 27 ÷ 6.41 = 4.21 tons. Henee, roughly, 6½ cubic feet weigh a ton, and a cubic yard weighs 4¼ tons. The ordinary ore, mixed more or less with rock, will run from 6½ to 7½ cubic feet to the ton.
Magnetic iron ore is found in igneous and metamorphic rocks in lenses or in vein-like deposits; also in beds of siliceous ore in pre-Cambrian rocks of sedimentary origin.
Ilmenite or Titanic Iron Ore: FeTiO3
Ilmenite or Titanic Iron Ore are in Color, iron-black, very much like magnetite; powder, black to brownish red; luster, near-metallic; H = 5 to 6; G = 4.5 to 5. Composition: iron, 36.8%; titanium, 31.6%; oxygen, 31.6%. Very slightly magnetic, and thus distinguished from magnetite. Titanium in iron ores is objectionable, causing difficulties in smelting; but since there are large deposits of magnetite high in titanium, though otherwise pure, these difficulties will, in time, be overcome, and these ores will then become valuable. Ilmenite is valued as an ore of titanium, selling a ton of ore on the basis of 59% to 60% titanic oxide, TiO2. Ilmenite is used for making titanium metal, as well as ferro-titanium, employed in making steel, and for making titanium-white, a white pigment used for paints with great covering power and durability. Titanic oxide, made from ilmenite, is used to give a yellow color to enamel. In Norway, a number of paints (red, orange, yellow) have been manufactured by roasting titanic iron ores.
Found in gabbro, diorite, syenite, etc., often mixed with more or less magnetite. Sometimes altered to a dull-white substance, leucoxene, of variable composition, but sometimes of the same composition as titanite or rutile.
Franklinite
Franklinite is an oxide of iron, zinc, and manganese; has no simple composition formula, as the proportion of the metals vary. Color, iron-black; looks much like magnetites but is not so strongly magnetic; powder, reddish brown or black; H=5.5 to 6.5; G=5.07 to 5.22. An average analysis of franklinite shows it to contain iron, 45.53%, manganese, 10.29%, zinc, 18.70%, oxygen, 24.48%. It is used as an ore of zinc, and the residue as an iron ore; its value is not quoted on the market. Crystals, in the cubic system, octahedrons, like those of magnetite. Found with other zinc ores.
Hematite: Fe2O3
Hematite is of Color, dark steel-gray or iron black; powder, red or reddish brown; luster, metallic, sometimes dull; H = 5.5 to 6.5; G = 4.9 to 5.3. Hematite deposits are often compacted powder or partly so. The ore in such case is red. Red ocher is fine-grained hematite loosely compacted; reddle and red chalk are the same, with more or less clay; specular hematite is in brilliant black crystals (from speculum, a mirror); when the crystals are small and thin, like scales of mica, the mineral is micaceous hematite; clay ironstone is a hard, brownish-black to reddish-brown mixture of hematite and clay or sand, etc.; kidney ore is hematite in rounded masses, which break with a smooth fracture and more or less radiating structure.
All varieties of hematite give a red powder. Earthy and micaceous varieties seem much softer than the mineral really is, because the test results in separating the grains already formed; it does not give a correct idea of the hardness of the individual grains. Sometimes hematite is slightly magnetic. The pure mineral contains iron, 70%, oxygen, 30%. As mined, iron ores are more or less mixed with rock (gangue), which lessens the percentage of iron; an ore is considered rich if it carries 60% of iron, and the average per cent of iron in the ores, as fed into the furnaces is not much above 50. Poorer ores are often improved (beneficiated) by washing, which removes the lighter material, or by magnetic concentration (in the case of low-grade magnetite).
Value of hematite is cheap for non-bessemer ore (that is, high in phosphorus), 51½% iron, and more for bessemer ore, 51½% of iron. Found in rocks of all ages, in vast quantities in sedimentary rocks, and in smaller masses in igneous rocks.
Limonite: 2F2O33H2O
Limonite is in Color, brown or black, the compact, hard ore is dark brown, sometimes nearly black; when soft and earthy (bog ore and yellow ocher), it is brownish yellow or yellow; powder, yellow or yellowish brown; luster, non-metallic, near-metallic, or earthy, sometimes silky; H = 5 to 5.5, but the earthy varieties crumble easily when tested with the point of a knife, and thus may seem quite soft; G = 3.6 to 4; not crystalline. The composition is the same as that of hematite, with the addition of water (H2O). The formula as written above shows that there are 2 (56 x 2 +16 x 3) = 2 x 160 = 320 parts of hematite and 3 (1 x 2 + 16) =54 parts of water; and the proportion of the iron in the ore is 2 x 56 x 2/320 + 54 = 224/374 = .599 = 59.9%. Limonite is found in large deposits of hard ore, and also as a loose covering of the bottoms of shallow lakes and bogs.
Goethite: Fe2O3H2O
Goethite is like limonite, but is crystalline; it is a little heavier than limonite (G = 4 to 4.4), and is sometimes reddish in color; powder, brownish yellow to yellow. Turgite, 2F2O3H2O is like limonite in appearance, but gives a red powder, like hematite. In composition, it lies between the two, having some water, but less than goethite.
Limonite is peculiar among valuable minerals in the fact that in some places it is being deposited fast enough to renew the supply in a few years after it has been exhausted. It is said that at Radnor Forges, in Quebec, the bottoms of the shallow lakes were cropped every seven or eight years. As iron ores, limonite, goethite, and turgite have values similar to those of magnetite and hematite. Ore such as was mined originally at the Helen Mine, Michipicoten District Ontario, and more recently at Steep Rock Lake, Ontario, and on the vast new iron range in Labrador and New Quebec, ranks with the best hematite.
Chromite: FeCr2O4
Chromite of Color, iron black to brownish black; powder, brown; luster, metallic or near-metallic; sometimes slightly magnetic; looks like magnetite, but is usually duller in luster ; seldom strongly magnetic; H = 5.5; G = 4.32 to 4.57. Composition, oxide of iron and chromium; the latter metal gives the mineral its value for the manufacture of chromates and similar chemicals, and paints (chrome yellow, chrome orange, chrome red, chrome green). The metal chromium is used in making the chrome-nickel alloys mentioned below; also, in making stellite alloys, mentioned under smaltite. Value of ore long ton for ore containing 48% to 51% of chromium oxide, provided the iron content is low. High iron reduces the value. Chromite is found in serpentine rocks.
Stellite Alloys
Since Ontario produces an appreciable part of the world’s cobalt ores and Quebec has deposits of chromite, Canada has the raw materials for making stellite alloys, manufactured at Deloro, in eastern Ontario, since 1915. While the original stellite contained only cobalt and chromium, a third metal, tungsten, has been added, the percentages being: cobalt, 50% to 60%; chromium, 40% to 30%; tungsten, 20% to 8%. The alloy is used for the manufacture of machine tools, cutlery, surgical and dental instruments, evaporating dishes, annealing dishes, ornamental work, valves, plumbing fixtures, pens, and combs. The silvery white color, stainlessness, and great hardness (some varieties are harder than quartz) fit it for these uses. By using stellite tools, the speeds of machine tools have been increased as much as 50%. More recently its heat resistance has made it useful in parts of the jet engine.
Nichrome
Nichrome is an alloy of nickel, chromium, and iron. It resists high temperatures and is used for the heating elements of toasters, irons, furnaces, etc.
Stainless Steel
Iron alloyed with chromium in sufficient proportion resists rusting and other corrosion better than ordinary steel. The resisting power is particularly marked when about 20% of chromium is used, and is still greater when nickel is added as well as chromium. A good deal of cutlery is now made of high-chromium steel. It is also used in parts of machinery that have to withstand corrosion. Plating with chromium is commonly used for automobile finishing and for bearings.
Tinstone or Cassiterite: SnO2
Tinstone or Cassiterite are of Color, brown or black, sometimes red, gray, yellow, or white; pure oxide of tin is white; powder, brownish, gray, or white; luster, adamantine (like diamond); H = 6 to 7; G= 6.8 to 7.1; crystals, tetragonal; when in crystals, the luster is sometimes very brilliant, but other samples are dull. Wood tin has a somewhat fibrous structure, and mixed brownish shades, like dry wood. Toad’s eye tin is similar to wood tin.
Stream tin is pebbles and grains of tinstone, as found in beds of streams and on shores.
Tinstone is the common ore of tin; its value is not often quoted; the value of the ore can be judged from the percentage of tin it contains. Tinstone is found in veins in granite, gneiss, mica schist, slaty schist, quartzite, and quartz porphyry. It is stated that in all productive tinstone areas, the ore is associated with a peculiar granite called greisen, which is composed mostly of quartz and white mica. The greater part of the world production of tin ore is stream tin.
Corundum: Al2O3
Corundum is of Color, commonly gray or brown, also blue, red, yellow, and nearly white; pure aluminum oxide is white; powder, white or gray; luster, like diamond or glassy; H = 9; G = 3.95 to 4.10; fine, clear blue crystals are valuable as gems, and are then called sapphires; rubies are crystals of red corundum. Crystals, in hexagonal system; parting, good, but no true cleavage. Emery is usually black, fine-grained corundum, with magnetite or hematite; is sometimes hercynite, FeAlO4.
Corundum being harder than quartz is valuable as an abrasive (material for grinding and polishing). Artificial corundum is now made by an electric furnace process from non-crystalline aluminum oxide, which can be made cheaply from bauxite, and being of the same composition as corundum, requires only to be crystallized. Corundum is found in nepheline-syenite and similar rocks; also in peridotite.
Bauxite: Al2O3·2H2O
Bauxite is of Color, whitish, grayish, yellow, brown, red; powder, yellowish, brownish, or white; G = 2.55; earthy, often found with round masses like small eggs, and therefore called oolitic in structure. Composition is the same as corundum, with water in addition; but generally more or less limonite is mixed with the bauxite, causing the brown color. Its principal use is as an ore of aluminum. It is first purified, then fed into a bath of fused cryolite, in which aluminum oxide is soluble; the aluminum is deposited by a current of electricity. Alundum is artificial corundum made from bauxite by heating in electric furnaces. Bauxite bricks are used for furnace linings.
Bauxite is found in limestone and around the margins of clay deposits, in pockets or as “blanket” deposits.
Pyrolusite: MnO2
Pyrolusite of the Color, iron-black, dark steel-gray, sometimes bluish; powder, black or bluish black; luster, metallic; H = 2 to 2.5; soils the fingers when handled; G = 4.37 to 4.86; usually in long crystals, radiating or columnar, sometimes granular; composition, manganese oxide; it is often called manganese ore. Used in the manufacture of certain chemicals, particularly permanganates, but mostly in steel manufacture. Manganese steel, or Hadfield steel, usually contains 12% to 15% of manganese. It is very tough, and is used for jaws and other wearing parts of rock crushers and similar machinery; for railway frogs, crossings, and curves; for mine-car wheels, trolley wheels, linings of ball mills, burglar-proof safes, etc. It lasts much longer than ordinary steel, because it is not only tough but becomes tougher with use. Manganese is added in small quantities to ordinary steel up to 1%; more than this makes the steel brittle; but when the amount of manganese is increased to 7%, the properties of Hadfield steel begin to appear. Ferro- manganese is an alloy of iron and manganese, containing from 40% to 80% manganese; it is used in making manganese steel. Spiegeleisen is pig iron very high in manganese, 15% to 30% ; it is used to give the required amount of manganese to ordinary steel.
Pyrolusite and other manganese ores are sold on the unit system, 90 to 95 cents per unit, the unit being 1% of manganese in a ton of ore. Pure pyrolusite contains about 65% of manganese. The best ore is used in chemical manufactures. Pyrolusite and other ores of manganese are found in limestone, shale, sand- stone, etc., in veins and bunches; sometimes in quartzite, chert, and jasper; occasionally in bunches in clay.
Psilomelane or Hard Manganese Ore
Psilomelane or Hard Manganese Ore are in Color, black, bluish, or brownish black; powder, brownish black, luster, silky or dull; H = 5 to 6; G = 4.1 to 4.7; composition, oxides of manganese with potash or baryta, with sometimes water; generally impure. This is a common ore of manganese; often found with pyrolusite, but distinguished from it by its greater hardness. Wad, or bog manganese is a brown, soft, earthy manganese ore, variable in composition ; it looks like bog iron ore, but is often darker, and is often found with pyrolusite. Asbolite, or earthy cobalt ore, resembles wad, but contains cobalt, sometimes enough to make it a cobalt ore. Lampadite is a variety of wad containing 3% to 13% of copper.
Braunite
Braunite are of Color, brownish-black to steel-gray; powder the same; luster, near-metallic; H=6 to 6.5; G=4.75 to 4.82; crystals, tetragonal; cleavage, perfect; composition, oxides of manganese with some silica. This is an ore of manganese, often found with other manganese ores. Hausmannite, Mn3O4, is a similar mineral of brownish black color; powder, chestnut-brown; H =5 to 5.5; G=4.8.
Zincite or Red Zinc Ore ZnO
The Color of Zincite or Red Zinc is red to brownish red; powder, orange-red; luster, like diamond; H=4 to 4.5; G = 5.43 to 5.70; crystals, hexagonal, like quartz; cleavage, perfect; composition, zinc (about 80%) and oxygen. Pure oxide of zinc is white; the color of the mineral is caused by a little hematite or oxide of manganese. Zincite is an ore of zinc, but is chiefly used in the manufacture of white zinc paint. For value, see Zinc Blende.
Cuprite or Red Copper Ore: Cu2O
Colors of Cuprite or Red Copper Ore are of various shades of red sometimes almost black; powder, brownish red, shining; luster, somewhat metallic or earthly; H = 3.5 to 4; G = 5.85 to 6.15; crystals, in cubic system, usually octahedrons; cleavage, fair. Often has green covering of malachite; often looks like hematite, but is softer. Contains nearly 80% of copper. Found sometimes with the commoner ores of copper.
Rutile (M 82): TiO2
Rutile has a Color reddish brown or red, sometimes yellowish, bluish, violet, black, or grass-green; powder, pale brown; luster. somewhat metallic; H=6 to 6.5: G=4.18 to 4.25 (for a black variety, nigrine, G =5); crystals, in tetragonal system, like those of tinstone; cleavage, distinct; composition, titanium oxide, with a little iron, sometimes up to 10%. For uses, see Ilmenite. Value, is good for a pound for ore containing 94% titanium oxide (TiO2). Found in granite, gneiss, mica slate, and syenite; sometimes in crystalline limestone and dolomite.
Quartz: SiO2
Quartz goes from colorless when pure to yellow, red, green, brown, blue ,purple, black; powder, usually white, sometimes of a pale color corresponding to that of the impure varieties; luster, glassy (vitreous); H = 7; G — 2.6 to 2.66; crystals, in hexagonal system; no cleavage; composition, Silicon oxide. Substances of this composition are often called silica.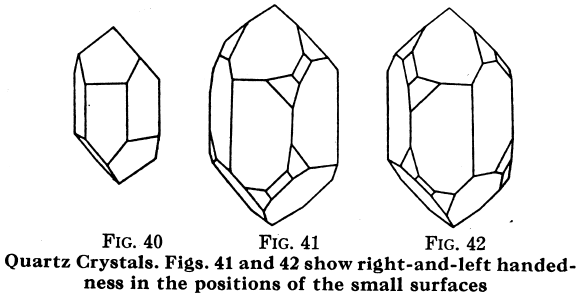
Varieties of Quartz
- Plainly crystallized (pheno-crystalline), including ordinary white quartz as seen in granite, quartz veins, etc., rock crystal (Cape diamonds, Brazilian pebbles, etc.), amethyst (purple of violet blue), rose quartz, false topaz (yellow), cat’s eye, tiger-eye, aventurine (spangled with scales of mica, hematite, etc.), and many others.
- Not plainly crystalline (cryptocrystalline or partly amorphous). Chalcedony, somewhat waxy luster, transparent or translucent; white, grayish, blue, brown, black. Carnelian, clear, red chalcedony. Chrysoprase, apple-green chalcedony, color due to nickel oxide. Bloodstone, green, with small spots of red jasper. Agate, chalcedony banded, clouded, or mossy, in various colors. Onyx, like agate, but in even layers of white and black, white and red, and so on; used for cameos. Sardonyx, like onyx, but including layers of carnelian. Siliceous sinter, irregularly cellular quartz deposited from siliceous hot springs, etc. Flint, somewhat like chalcedony, but not so clear and of dull colors, usually gray, smoky brown, and brownish black. Hornstone, like flint, but more brittle and with a more splintery break. Chert, any impure flinty rock, including hornstone, jasper, etc. Touchstone, or lydian stone, a velvet-black siliceous stone or flinty jasper. Jasper, impure, opaque, colored quartz; commonly red, also, dark, green and grayish blue. Quartzite, quartz sandstone, quartz conglomerate, flexible sandstone (itacolumite), and buhrstone, are rocks composed mostly of grains or pebbles of quartz.
- Opal, SiO2 with water in varying proportions. Color, white, yellow, red, brown, green, gray, blue, sometimes with a fine play of colors (precious opal); H = 5.5 to 6.5; G = 1.9 to 2.3; not crystalline. Tripolite, infusorial earth or diatomite, is a deposit of microscopic shells; is similar to opal in composition: though soft and earthly looking, it is in very small grains, hard enough to scratch glass. Tripoli is a somewhat similar material of different origin, being a residue left by the weathering of chert and other siliceous rocks. It is not likely to be found in glaciated countries.
Ordinary vein quartz, white sandstone, and quartzite are used for lining smelting furnaces, and in the manufacture of glass and pottery; but for these latter uses, pure quartz sand and flints are commonly used. For all these purposes the quartz is valued at from $15 to $50 per ton; very pure, white quartz when finely ground is used for wood filler, to make the wood take a high polish, and is sold by the ton. Tripolite, or infusorial earth, is used for polishes, packing for boilers, heat and sound insulators, filters, and as an absorbent. The uses of tripoli are somewhat the same as those of diatomite, but it is also used to mix with soap and paint, and as a filler in manufacture of rubber, for foundry facings, etc. The ground and purified material sells for a good price. Some varieties of natural quartz, such as sand and sandstones, have well-known uses in building.
Quartz crystals, when large, colorless, and flawless, are sometimes ground into spheres for ornamental purposes, a sphere 5½ inches in diameter. Crystals for fusing sell well. Specially clear crystals for optical purposes sell for alot. Clear, finely colored crystals (amethysts) are classed as jewels. The finer specimens of agate, carnelian, bloodstone, onyx, etc. are also of considerable value. Large sound pieces of ordinary agate are of value for making agate mortars, used in chemical laboratories.
