Table of Contents
- Cyanide Recovery OR Cyanide Regeneration
- Principles of Cyanide Regeneration
- Gold-copper Residue Treatment
- ION EXCHANGE USING SYNTHETIC RESINS
- Handling Waste Cyanide Solution
- Gold Chlorination Processes & Methods
- Bromocyanide Process
- Carbon Cyanidation
- Cyanide Regeneration Methods
- Crosse’s Method of cyanide regeneration
Cyanide regeneration offers a practical means of overcoming the otherwise heavy cyanide consumption frequently encountered in the treatment of gold and, especially, silver ores, where cyanicides (cyanide consuming minerals) are present.
Miscellaneous processes used for the extraction of gold and silver values by hydrometallurgical means are also discussed in this chapter. These include the carbon-cyanidation, the bromocyanide, the ammonia-cyanide, and chlorination processes. Though some of these are not used commercially today, they are of interest both historically and because they contribute to the sum of our general technical knowledge, out of which new and improved processes for the future may be developed.
A. J. Clark, metallurgist, once made the significant remark that every process, no matter to what extent it might be regarded as impractical, should be reviewed every 5 years with the thought in mind that improvements in equipment and techniques might possibly justify its revival as a working scheme.
In keeping with this viewpoint, we have included in the present chapter brief descriptions of certain processes generally, regarded as out of date and superseded by more modern and technically efficient methods, for the fact remains that in many cases the cost of treatment by our improved present-day metallurgy is often discouragingly high and in other cases it is not possible to treat the ore at all.
If the technology of ore treatment is to make a steady advance, it is imperative that research men and engineers keep an open mind in the matter of reappraising the obsolete processes and also what may sometimes appear to be the new-fangled ideas of overenthusiastic inventors. Both may well contain the germs of ideas that lead to important process developments.
Cyanide Recovery OR Cyanide Regeneration
With regard to regeneration of cyanide, J. E. Clennell said in The Cyanide Handbook:
Since the main cause of cyanide consumption is the formation of soluble double cyanide or complex cyanogen compounds of the base metals, and since solutions highly charged with such compounds are more or less inefficient as solvents of gold and silver, it has been suggested that the cyanogen in such liquor might be recovered in the form of simple alkali cyanides, by treatment with suitable chemicals. This has been carried out in practice in some cases, but generally speaking the cost of chemicals, power, labor, and other charges required for such treatment outweighs the advantage gained by it.
The latter part of the foregoing statement may be questioned by some, but although the few regeneration plants at work report a saving in cyanide and an improvement in the treatment, methods for the recovery of cyanide have not been generally adopted.
Under normal operating conditions the mechanical loss of cyanide discharged with the tailing is an important factor in cyanide consumption. This loss results from imperfect washing, which in turn is the result of limiting the water wash to the amount of water necessary to maintain a balance of plant solution. This difficulty can be practically eliminated by using solution from which the cyanide has been removed to extend the washing period, according to W. E. Crawford of Fresnillo, Mexico.
In Handbook of Ore Dressing, 1928 ed., by A. L. Taggart, R. C. Canby said:
Precipitation of gold and silver from cyanide solution by means of zinc or aluminum results in regeneration of cyanide, probably not in the form of alkali cyanide as originally added but in a form in which an equivalent amount of effective cyanide ion is present.
The common method for regeneration of cyanide is by acidulation of the solutions. All or part of the cyanogen is converted thereby into hydrogen cyanide, which is fixed by an alkali (generally lime) and returned to the cyaniding system. This is the Mills-Crowe process, the principle of which is described by C. W. Lawr in T.P. 208, A.I.M.E., 1929, in which he also gives a selected list of 38 references to regeneration:
[
Principles of Cyanide Regeneration
The solution, be it a weak wash or a foul solution, is made acid by bringing it into contact with sulphur dioxide. The acidified solution is then transferred to a closed tank in which air and solution are brought into intimate contact. The air leaving the tank charged with hydrogen cyanide is then passed to another tank in which it is mixed with an alkaline solution, the latter absorbing the HCN and leaving the accompanying air clean for reuse in removing more HCN from the acidified solution.
The extent to which the acidified solution will become impoverished of its cyanide will depend upon the acidity, the amount of air brought into contact with the solution, and the quantity of residual HCN left in the air after the latter has passed the absorbing apparatus. (The system is closed so that the same air is used repeatedly.)
Of course the amount of air required will depend on how efficiently it is utilized, but where other conditions are equal, it may be stated that the amount of cyanide removed from a given volume of solution increases with an increase in air, and, further, an increase of air will do more good or a decrease will cause poorer results than almost any other change that could be made in the plant. (By increase in air is meant an increase in the velocity of that being circulated.)
The impoverished acid solution may be wasted or used as a water wash on the filters, either before or after filtering, depending on whether the solution contains enough silver or copper to pay for its removal. If the spent solution does not contain any silver, but much copper, it is doubtful whether it could be used as a filter wash before filtering, because the slimy nature of the precipitate would affect adversely the filter leaching rate.
The Mills-Crowe cyanide-recovery process is applicable only in plants of rather large tonnage and best on solutions resulting from the treatment of silver ores or those containing appreciable amounts of copper.
Cyanogen in mill solutions exists chiefly as free alkali cyanide, as zinc and copper double cyanides, and as sulphocyanide and ferrocyanides. From the free cyanides and zinc double cyanide, substantially complete recovery of the cyanogen is easily effected. Part of the cyanogen combined with copper is readily recovered; regeneration of the remainder, with the cyanogen existing as sulphocyanide and ferrocyanides, requires special treatment which well may be justified in large plants or in plants where unusually strong solutions are employed.
Cyanide Regeneration at Pachuca, Mexico
At Pachuca treatment of the silver ore, which carries some copper, and gold involves a high cyanide consumption, which is reduced by cyanide regeneration. After the usual washes of the filter cake, including a water wash passing to mill make-up, the cake is given a further wash of 20 min., and the values in cyanide and precious metals contained in this solution are removed in the regeneration plant, the cyanide being returned as a gas and absorbed in the mill solution, while the silver and gold and quantities of copper are recovered in the form of a precipitate, which is shipped to a smelter.
The plant treats 3800 tons per day of a solution composed of 1000 tons of barren solution and 2800 tons of water wash from the filters.
NaCN Regeneration consists of:
- acidifying the solution with sulphur dioxide to neutralize lime and convert the cyanides to hydrogen cyanide,
- vaporizing the hydrogen cyanide from this solution by means of a large volume of air,
- absorbing the hydrogen cyanide carried by this air in the regular mill solutions,
- adding zinc dust to precipitate the gold,
- recovering the gold-silver-copper precipitate by filtration.
The sulphur dioxide gas used in the process is made in a rotary sulphur burner. It is brought into contact with the cyanide solutions in an acidifier. The HCN formed in the acidifier remains in the acid solution until removed in the dispersers. This gas is dissolved in the solution, and although it can be removed fairly easily, it is fixed sufficiently in the acidified solution so that, upon passage through a weir box 6 ft. in length open to the atmosphere under normal conditions, no loss of HCN can be detected by silver nitrate titration of the solution as it enters and leaves the weir. At times, an odor of HCN is noticeable, indicating a slight loss.
The HCN is removed from the acidified solution by bringing a large volume of rapidly moving air into contact with the acidified solution spread over a large surface. Solution-surface exposure is obtained by grids and spraying devices. At least 15 cu. ft. per min. of air per ton of solution treated in 24 hr. is required.
The HCN removed from the acidified solution then is absorbed by an alkaline plant solution in horizontal absorbing towers in which the solution and the air containing the gas are brought into close contact.
R. R. Bryan, general superintendent of mill at Pachuca, writes:
The regeneration of the quantity of barren solution was undertaken to keep the plant solutions reduced in certain constituents which, when allowed to build up, caused poor extractions and poor settling. Copper is one of these deleterious constituents, and although unknown, we suspect that there may be others. We endeavor to keep the copper content of the thickener overflow below 80 grams copper per ton of solution. With this bleeding of 1000 tons barren solution per day there has been a great improvement in settling and former periods of unusually low extraction have been avoided.
Another change in cyanide regeneration has been the introduction of entirely automatic acid control. This is accomplished by means of a Beckman pH meter and a Bristol potentiometer controller which operates the air valve on the sulphur burners. The correct pH for optimum results depends on the constituents of the particular solution. For filter washes it is pH 5.6, but with the addition of barren we use pH 5.1.
The difference in pH required for different solutions appears to be due to different amounts of double zinc cyanides which they may contain. The double zinc cyanide requires a higher pH for its complete regeneration than do the simple cyanides.
The introduction of automatic pH control has been a major improvement in our cyanide regeneration. The amount of sulphur required has been reduced, the tails are lower, and less trouble from liming up is encountered. Before pH control, the regeneration of an considerable tonnage of plant barren solution was very difficult because of liming up. The use of pH control has greatly reduced this difficulty.
Cyanide Regeneration at HudBay Minerals
The operation of a cyanide regeneration plant in Canada is described by the mill staff in “Cyanide and Regeneration Plant and Practice at Flin Flon” (Trans. 49, C.I.M. and M. 1946) as follows:
From the storage tank following precipitation, barren solution is pumped to the Mills-Crowe regeneration plant for recovery of the cyanide, which is reused during agitation.
The elements of a single regeneration unit are shown in Fig. 60. They consist of:
- A dispersing tower.
- An absorbing duct.
- A blower rated at 36,000 cu. ft. per min. and 4-in. water gauge.
- A centrifugal pump for dispersed solution.
- A centrifugal pump for absorber solution.

Acidified barren solution enters the top of the dispersing tower, where it is distributed across the tower section by three launders. The solution drops through the grid-packed tower countercurrent to an air stream discharged by the blower and into a sealed sump at the tower bottom. From the sump, a centrifugal pump transports the solution to the next unit.
The air stream in its passage up the tower sweeps the hydrocyanic acid gas, liberated by the action of the acid, into an air duct leading to the absorber. This is a tunnel about 40 ft. long and 4 by 6 ft. in section. One end joins the air duct from the disperser, and the other end forms the blower inlet. The tunnel floor, over which absorber solution flows, slopes at ¼ in. per ft. to a sealed sump. From the sump, a centrifugal pump elevates absorber solution to a storage tank feeding the absorbers and agitators. Six rotor sprays are installed at intervals across the tunnel, about 3 in. above the floor. The rotors are 12 in. diameter by 4 ft. long and are driven at 900 r.p.m. by 3-hp. motors. The adjustment of a butterfly weir at each rotor controls the depth of its immersion and the fineness of the mist in the tunnel atmosphere. A frame of 30 metal louvers located across the tunnel near the blower intake protects the fan from lime-scale coating by minimizing the mist entering the blower.
The air stream carrying hydrogen cyanide gas enters the absorber at its junction with the disperser air duct. It is drawn through the mist of absorber solution, a weak lime slurry. The cyanide gas reacts with the absorber mist and remains in solution as calcium cyanide, while the other gases pass on into the blower. The blower discharges into the disperser tower, completing the gas cycle.
The entire plant consists of:
- Four dispersing units.
- Four absorbing units.
- An absorber storage tank feeding the absorber units and cyanide agitators.
The dispersing units operate in series. Barren solution is acidified with waste sulphuric acid from the electrolytic section of the zinc plant in a lead-lined box ahead of unit 1. Acid addition is controlled at this point to give a residual acidity of 1¾ to 2 lb. acid per ton of solution at the last disperser. A minimum residual strength of 1¼ lb. per ton is required for good dispersion. From the last disperser, the solution is pumped to the copper sulphate plant for further treatment.
The absorber units operate in parallel. From the storage tank, the absorber solution flows by gravity to the high end of each of the four units. It becomes enriched in cyanide during its passage through the absorber and is pumped back to the storage tank by 5-in. pumps.
An 18- by 18-ft. wood-stave tank equipped with a 48-in. ship-type impeller is used for absorber solution storage. From this reservoir, the final regeneration plant product is drawn for reuse in the agitators and circulates to each absorber unit. The alkalinity of the solution is maintained at 1 lb. CaO per ton by addition of plant lime slurry. The draw-off to the agitating section is replaced with water. A portion of the flow to the agitators is used to dissolve and transport the raw cyanide addition.
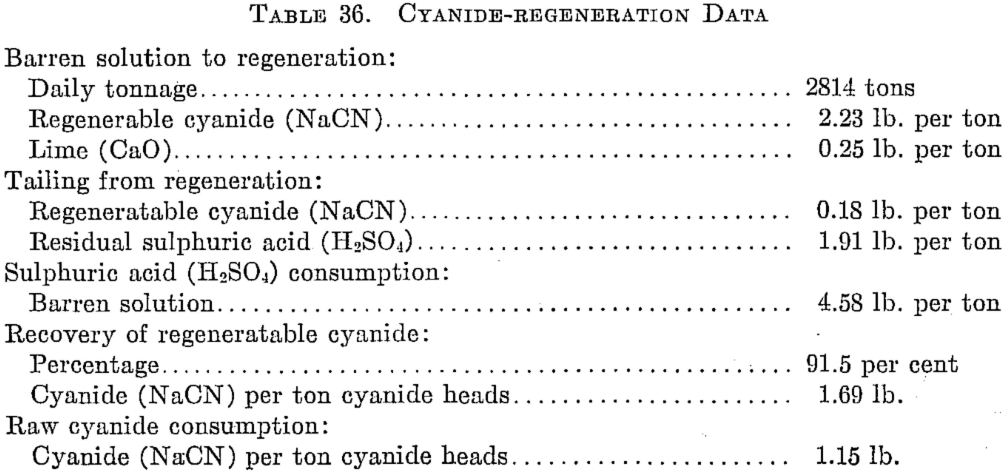
The regeneration operation for a year is summarized in Table 36.
Leaver and Woolf Process, U.S. Bureau of Mines. In T.P. 494, “Copper and Zinc in Cyanidation. Sulphide-Acid Precipitation,” the authors E. S. Leaver and J. A. Woolf describe a process for the regeneration of a large part of the cyanide combined with copper and zinc, in fouled mill solutions. This process, which as far as we know has not yet been used on commercial scale, involves the addition of a soluble sulphide to the solution followed by acidification in a closed reactor to pH 5 to 6, whereby the zinc and copper are thrown down as sulphides and up to 80 per cent of the combined cyanide regenerated. After removal of the precipitate by filtration lime is added to the filtrate to “fix” the HCN regenerated, and the solution is ready for reuse.
The silver would normally come down with the copper and zinc precipitate, but it may be separately removed as silver sulphide if desired before acidification. Only a part of the gold is precipitated; the rest must be recovered by the usual zinc-dust treatment.
The process must, of course, be carried out in a carefully closed system to avoid the hazards of HCN asphyxiation, and it has the disadvantage that sulphocyanates, cyanates, etc., are not broken up. It is stated that the precipitated sulphides are readily filtered and contain no cyanide, but the exact manner of handling and retreatment for extraction of precious metals is not described.
This technical paper is, however, of special interest to those concerned with the problem of soluble copper and zinc in cyanide solutions.
General Engineering Company Process. In this process the free cyanide and free lime as well as any copper, zinc, or silver compounds are precipitated by the addition of chemically equivalent amounts of zinc sulphate. The precipitate, following filtration, is treated with acid for regeneration and recovery of CN by the usual stripping and absorbtion methods, and the filtrate treated with zinc dust or other suitable precipitant for recovery of the gold.
The cyanide tied up with the copper and silver is lost in the proposed smelting method for recovering the metals, but the process has the advantage that only a small bulk of precipitate is acidified as against the whole volume of solution in other processes, and it would also appear that a considerable part of the acid ZnSO4 could be reused after neutralization with lime.
Australian Practice. At the New Occidental Gold mines N.L. Cobar, New South Wales, Australia, solution containing copper from the direct cyanidation of ore containing pyrrhotite and chalcopyrite (from 0.1 to 0.3 per cent copper) is treated by a batch process.
When the working solution increases in copper content above 0.03 per cent, the double cyanide of copper, Cu2(CN)2 2NaCN, is precipitated by strongly acidifying the solution with H2SO4. This brings down a bluish- black precipitate of cuprous cyanide which is allowed to settle, the clear solution being decanted off into an agitation vat where lime is added to give a protective alkalinity of approximately 0.1 per cent CaO.
By this method profitable regeneration of the free cyanide from the double cyanide is accomplished and the copper content of the plant solutions kept below 0.03 per cent copper. It was found in practice that, when the copper content increased above this figure, dissolution of gold was reduced considerably.
The low-grade copper precipitate produced in the process is run to waste from the settling tank, since shipping it to a smelter proved uneconomical.
Gold-copper Residue Treatment
H. B. Wright stated in E. and M.J., May 5, 1923, that regeneration of cyanide from cupriferous cyanide solutions is profitable, easy of application, and made possible the treatment of a dump of refractory residue in New South Wales, Australia. This material contained about $40 gold per ton and 0.1 per cent copper in the sand and 0.33 per cent in the slime. While treating more than 10,000 tons of this mixture, half of the cyanide was regenerated and about 1 lb. per ton copper was recovered.
To neutralize acidity, 4 lb. lime was added to each ton of sand going to the leaching vats; 2 tons of cyanide solution per ton of sand was used during the 14 days treatment. But as it is essential for regeneration to deprive solutions of alkalinity or free cyanide, the sump solution titrating 0.06 per cent NaCN and 0.04 per cent CaO was applied to the 70-ton sand vats, a half ton to each ton of sand, preceding the neutralization by lime. The solution draining away was neutral to phenolphthalein and was pumped to the regenerating part of the plant for subsequent treatment.
It was found best, by repeated tests, to apply one case or 224 lb. cyanide all at once to-a vat containing 70 tons sand. The next step was to pump regenerated solution of 0.14 per cent NaCN and 0.10 per cent CaO strength over the cyanide placed on top of the sand, which dissolved all of the new cyanide required and raised the strength to 0.40 per cent. This solution was circulated by pump for 8-hr. periods over two days. Then it drained to the zinc boxes, having 0.15 to 0.20 per cent free cyanide.
Precipitation on zinc was for the richer and stronger solutions only, and 80 per cent precipitation of the gold was considered good work under the conditions of treatment. Calcium sulphate mud was a nuisance in the. boxes. The gold-bearing sludge was so refractory that it was sold to a smelter.
Precipitation of gold from the weaker solutions was accomplished by sulphuric acid. Copper was present in the solution as the double cyanide Cu2(CN)2 2NaCN and was precipitated in the paddle agitator mentioned in the next paragraph by means of sulphuric acid. The dried precipitate assayed 60 per cent copper, 70 oz. gold, and double that of silver.
Cyanide Regeneration Process
The solutions neutralized and freed from active cyanide, as already mentioned, were eventually pumped to an elevated agitating tank of 50 tons capacity. Enough sulphuric acid was then added to throw down as cuprous cyanide, Cu2(CN)2, all of the copper present. Each charge of solution required from 7½ to 14 lb. per ton. The agitator, from which an unpleasant odor arose, was then stopped, and the cuprous cyanide precipitate allowed to settle for 1½ hr. The clear liquor, charged with hydrogen cyanide, was decanted into a 50-ton Dorr agitator. Milk of lime then was added until the solution showed 0.09 to 0.13 per cent free CaO and 0.14 per cent free NaCN. Agitation was effected by pumping. The regenerated solution was then pumped into the head tank and reused in treatment, as described.
The Dorr agitator was cleaned of insoluble matter at periods of 3 to 6 months. The cuprous cyanide precipitate from the paddle agitator was allowed to accumulate during six charges, then was washed out and drained on a filter consisting of wooden slats, coconut matting, and sacking and finally dried and shipped to the smelter.
NOTE. Although no ill or serious results ever attended the use of this process at this plant, it should be used with caution because of the extreme danger of cyanide poisoning by hydrocyanic acid gas.
ION EXCHANGE USING SYNTHETIC RESINS
Ion Exchange
As an alternative to the use of activated carbon for the removal of gold and silver from cyanide solutions by the mechanism of adsorption, the application of ion-exchange methods using synthetic resins has been proposed.
Tests along these lines carried out by the U.S. Bureau of Mines on ore from Buckhorn mining district in Nevada. The ore was chosen because its very poor settling and filtering characteristics made conventional cyanidation impractical. It was ground to minus 100 mesh for the present tests. Only the plus 35- mesh fractions of the commercial resins were used, and both the anionic and cationic types were investigated. After a period of agitation with the cyanided ore pulp, the resin was separated by screening and washing on a 65-mesh sieve.
The gold and silver were then removed from the resin by eluting with sodium hydroxide, after which it could be used for subsequent adsorption, though with increasing loss of capacity with each cycle.
Summarizing the tests, the author states that about 78 per cent of the gold and 50 per cent of the silver in the ore were recovered by countercurrent adsorption and regeneration.
While the countercurrent method proved to be the best system, it was not practical to make use of the simultaneous dissolution and adsorption technique worked out by T. G. Chapman owing to the marked adsorption of free cyanide by the resins.
The anionic-type resins were found to be superior to the cationic type in the particular pH range and other conditions investigated, and about 25 lb. resin per ton of ore treated was required, though the actual consumption would presumably be only a small fraction of this, depending upon the undetermined amount of replacement required.
A 48-hr. leach was used, followed by adsorption times as short as 15 to 30 min. for 95.4 per cent gold and 79 per cent silver removal from the pregnant solutions. Sodium hydroxide, which removed close to 99 per cent of the precious metals adsorbed, was found to be the most efficient of the eluting and regenerating agents tested.
A more detailed investigation is required before the advantages and limitations of the use of ion-exchange resins, as compared with activated charcoal, can be evaluated. At the present time it would appear that charcoal is a cheaper material and one that is more resistant to mechanical abrasion and chemical deterioration.
Ammonia-cyanide Process
R. J. Lemmon points out that the original work on this process was clone at the University of Sydney. It was introduced into the United States by Bertram Hunt but has been used only on one or two minor operations, since closed down, in this country. Describing this process in a later issue of the same publication Lemmon says:
The employment of ammonia in excess of the amount of copper soluble in the cyanide solution, but in less amount than required to bring the total soluble copper into solution, has been found beneficial in increasing the gold solution, reducing cyanide consumption, and substantially reducing the amount of copper entering the gold precipitation unit. The mechanism of reactions includes the following:
2NaCN + Cu++ = Cu(CN)2 + 2Na+
and, 3CU(CN)2 + 4NH4OH = 2CU(CN)2 + 4H2O + [Cu(NH3)4].(CN)2
The cupriammonium cyanide dissociates into the complex radicle [Cu(NH3)4] and free cyanogen [(CN)2]. A further reaction probably ensues,
4CU(CN)2 + Cu(NH3)4 = 4NH3 . 2(CuCN)2
CU(CN)2 + (CN)2, dicuproso-cupric cyanide and cyanogen
It is believed that the (CN)2 liberated reacts with hydroxyl ions to form alkaline cyanides which then dissolve the gold. The ore to be treated should have a low permanent lime alkalinity, a weak solution of cyanide then added to dissolve some of the soluble copper, followed by slight excess of ammonia, and further lime added at the end of the treatment period to precipitate the ammonia-copper solution in the ore pulp. If the process has worked efficiently, the dicuproso-cupric cyanide should also be left as an insoluble in the pulp. Should the pulp contain interferent iron salts, a small amount of a soluble lead salt may be added before the addition of the cyanide. The method is applied in a similar manner to copper “oxides” such as malachite.
Where this method was used to treat a complex gold-bearing copper silicate ore in California, the cyanide consumption was reduced from 8 lb. per ton by direct cyanidation to 1 lb. per ton. The gold was precipitated in zinc boxes by the usual method.
Pretreatment to Remove Copper
Where sulphides of copper are the source of the trouble, their removal by flotation is frequently successful (the greater part of the chalcopyrite in the Noranda ore is removed by this method), but if the copper mineral is largely oxidized, chemical leaching methods may be indicated.
The use of either acid leaching or ammonia leaching is suggested. The latter usually involves a higher cost, whether recovery of the copper is or is not practiced, while the acid scheme suffers from the disadvantage that it is often difficult in practice to neutralize completely the residues from acid leaching and a rather carefully designed plant involving filtering and agitation equipment is required to ensure successful operation (see “Calcine Treatment at Rietfontein”).
If the copper is extracted with an acid solution, the most logical way of recovering the gold is with chlorine, and the most logical way of extracting the silver, is with a chloride solution. The various methods of chloridizing and of extracting silver by means of the CuCl3 solution alone or by the FeCl3 usually present are described (see “Chlorination ”).
Roasting Followed by Leaching
Where the above methods fail to solve the copper problem, roasting of the ore or concentrate followed by a water or weak acid leach before cyanidation is usually successful..
It might be mentioned in this connection that recent work at the Westport Laboratories of the Dorr Company using FluoSolids roasting technique indicates that this method can frequently effect extremely high conversion of the copper to the water- or weak-acid-soluble form. Any such improvement in the completeness of sulphatization will be of interest to those attempting to cyanide ores carrying copper, zinc, and other base- metal values which, unless removed before cyanidation, may render the cost in cyanide prohibitive.
Handling Waste Cyanide Solution
Gold Chlorination Processes & Methods
Bromocyanide Process
Carbon Cyanidation
Cyanidation and concentration of gold and silver ores
Cyanide Regeneration Methods
Arising out of the subject of the dissolving effect of the double cyanide is the question whether any part of this combined cyanide becomes available for dissolution purposes in the course of its circulation through the plant. Although the fact is doubted by some writers the indications certainly point to such a regeneration of free cyanide. This may take place from two causes, first, a precipitation of zinc by soluble sulphides formed during contact of the solution with the ore, K2S + K2Zn(CN)4 = ZnS + 4KCN, and second, a reaction of the free alkali on the double cyanide with the formation of an alkaline zincate and free cyanide, K2Zn(CN)4 + 4KOH = K2ZnO2 + 4KCN + 2H20. This reaction is no doubt reversible so that the dissociated cyanide would tend to recombine with zinc if any change occurred in the solution to disturb the equilibrium. Where the alkali employed is lime instead of caustic soda or potash there may be a formation of insoluble calcium zincate which renders the reaction non-reversible. In the operation of cyanide plants many metallurgists have observed that a saving in cyanide is effected by raising the lime content from a merely “protective” amount up to 0.1 % or 0.13% CaO. When the solution from the barren sump is thrown back to the mill and comes in contact with ore and fresh lime the free cyanide indicated by titration will often be found to rise in strength 40 or 50%, and its extractive efficiency will be increased in proportion to the increase of free cyanide as shown by the free cyanide determination. In a laboratory experiment made by the writer a solution of zinc-potassium cyanide was made up showing no free cyanide when tested by method No. 2, and having practically no dissolving effect on prepared Ag2S. An excess of lime was added to a portion of the solution, and after a few hours’ agitation it was filtered clear. A titration now indicated that about half of the combined cyanide originally present had been decomposed, and was recorded as free cyanide. This solution, when tested on prepared silver sulphide, had a dissolving efficiency equal to a freshly prepared cyanide solution of the same titration strength.
Crosse’s Method of cyanide regeneration
Some years ago a process of cyanide regeneration was patented by Andrew F. Crosse based on the principle of precipitating the zinc as sulphide by addition of sodium sulphide to the solution, K2Zn(CN)4 + Na2S = ZnS + 2KCN + 2NaCN. For this purpose the solution was to be heated to about 65 degrees C. (149 deg. F.), and the reagent added until a faint permanent trace of soluble sulphide was indicated in the solution on addition of a few drops of lead acetate. This, however, did not complete the process, because a difficulty arose due to the almost invariable presence in the stock solution of sodium zincate, Na2ZnO2: this zincate was acted upon by the sodium sulphide to form caustic soda; Na2ZnO2 + Na2S + 2H2O = ZnS + 4NaOH. The caustic soda, gradually building up in the stock solutions, attacked the zinc in the precipitation boxes, involving an additional zinc consumption and also causing such an evolution of hydrogen as to carry precipitate out of the boxes and send up the assay of the tails to a prohibitive extent. Crosse proposed to meet this condition as follows: when the caustic soda had accumulated to a dangerous extent in the stock solutions the use of Na2S was to be temporarily suspended and the reaction was to be effected by charging the tankful of solution with hydrogen sulphide generated from the zinc sulphide already filtered out and accumulated during the first stage of the process, causing the reaction, H2S + 2NaOH = Na2S + 2H2O. This part of the procedure, however, has so far proved impracticable on a working scale, and the process as a whole has not found any useful application.
It seems likely that the first stage of Crosse’s process might attain practical importance when aluminium is used as the precipitant, in the case of certain ores in the cyaniding of which zinc is detrimental to extraction of the silver, and yet which contain some zinc soluble in the cyanide solutions.
Several other methods of regenerating cyanide solutions have been proposed, chiefly based on the principle of precipitating the base metal contents with acids, but none of them has attained to any commercial importance.
