At the meeting of the local membership of the American Institute of Mining Engineers on December 14 last the question was asked by one of the speakers: “Why does the greased needle float on the surface of a tumbler of water and the wetted needle sink?” Did one or another of the experts present rise and say that it was due to ‘surface tension’ and then in a few well chosen words explain just exactly what ‘surface tension’ is? Nothing of the sort happened. The question was not only not answered, but it was unanimously avoided. It is a fair question, however, and deserves an answer.
The fact is that ‘surface tension’ is a misnomer. Tension is a stretching, whereas the phenomena in question are those of compression. In ‘surface tension,’ a bubble of air or a drop of water is pictured to the imagination as being actually of the form that it would have if it were contained in a film like that of a soap-bubble or a toy-balloon. That grasped, the substance of the bubble or of the drop is ignored and we are asked to occupy our minds only with the imaginary film. The reasoning appears to be: “There might be such a film, there must be, there is. Otherwise, we are not aide to explain it at all.” In what follows, I shall attempt to explain the phenomena discussed in terms of molecular attraction and of heat.
If you will fill a tumbler with water or other liquid and then continue carefully to pour in more; instead of running over the side, there will be a heaping up of the liquid in the tumbler and a rounding of the surface, the centre of the liquid being as much as a sixteenth or even an eighth of an inch higher than the rim of the tumbler. That is the phenomenon of your ‘surface tension’ pure and undefiled. The same phenomenon is seen when mercury is contained in a glass vessel, even when the vessel is only partly filled. Mercury does not wet glass, and where the liquid metal meets the side of the glass vessel the mercury is convex. Where, however, the tumbler is only partly filled with water the surface of the water is concave where it meets the inside of the tumbler, and the glass is wetted by the water.
In the Miami flotation case it was stated that surface tension is a force existing in the surface of a liquid that tends to draw the liquid into the form of a sphere, this being the most compact form that a given volume can assume and the form in which it presents the least surface. This is a lovely specimen of the logical fallacy known as post hoc ergo propter hoc. In surface tension, it was said in the Miami case, because the most compact form that a given volume can assume and the form in which it presents the least surface is a sphere, therefore the volume assumes that form and does so at the behest of surface tension; but as to the why and how, nothing was said, nor can they be imagined. It reads as if there had been a mass-meeting of the molecules looking to ‘preparedness.’ The molecule acting as chairman states the business before the assembled molecules: “Owing to the war in Europe and a hard winter coming on, the molecules must decide on some form that will be the most compact and which will present the least possible surface to an unsympathetic world. The sphere comes highly recommended. It is moved and seconded therefore that the molecules form a sphere. So ordered.”
The calculus proposition that two homogeneous spheres attract each other as if their masses were collected at their centres of gravity is as true as anything human can be. It is also time that in a single homogeneous sphere, if acted on by no outside force, the cohesive attraction of its molecules for each other will act radially toward the centre and form a sphere; and it is this radial attraction and not an imaginary film or a non-existent tension, that causes the phenomenon, and it is probably some similar molecular attraction that causes mineral flotation.
In James Clerk Maxwell’s article on capillary action, in the Encyclopaedia Britannica (11th edition), vol. 5, p. 258, he says: “Plateau, who made an elaborate study of the phenomena of surface tension, adopted the following method of getting rid of the effects of gravity. He formed a mixture of alcohol and water, of the same density as olive oil, and then introduced a quantity of oil into the mixture. It assumes the form of a sphere under the action of surface tension alone.” That it assumes the form of a sphere is granted. That surface tension does it is denied.
The toy-balloon has a place in a rational explanation of the phenomena under discussion; but the alleged film around a drop of water or around a bubble of air, or as the top layer of a body of water, like the film of a toy-balloon, has no existence in nature. The vendor of toy-balloons has each one of his gayly colored stock fastened by a string, which serves the double purpose of keeping the gas in the balloons and of keeping the balloons themselves down to earth. The free ends of the strings are brought to a common knot. There is a pull on each string along the hypothenuse of a right-angle triangle; this can be resolved into a vertical component, tending to make the balloon float off, and a horizontal component, tending to crowd the balloons together.
The same thing happens in surface compression. The water in the tumbler is subject to the cohesive attraction of its molecules, to the attraction of gravitation, and to heat. The water, if free from the attraction of gravitation, would tend to form a sphere, but gravitation causes it to conform to the shape of the containing vessel. Heat, by tending to drive the molecules apart, acts counter to the attraction of cohesion and their equilibrium fixes the specific gravity of the water, its bulk, and its state of aggregation—making it solid, liquid, or gaseous as the case may be. If gravitation be neutralized or be not opposed, the water takes a spherical form under the influence of cohesion, as is shown in raindrops, in Plateau’s experiment, and in drops of water on a hot stove, in conformity with the rule of homogeneous spheres.
Let us suppose each molecule of water in the tumbler to be free from the attraction of gravitation and in the form of a sphere, then the vertical section of the surface layer would look like this:
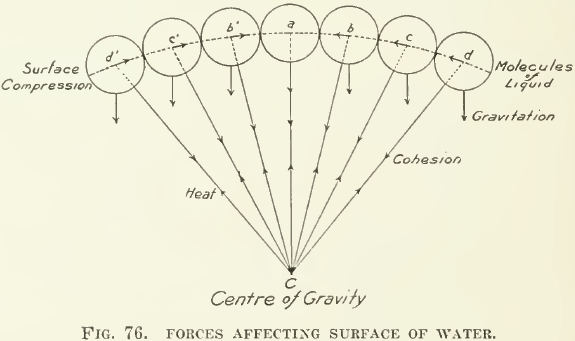
There you have the stock of toy-balloons with the strings connecting each with a common centre point. C is the centre of gravity of the water in the glass. The lines diverging from C show the directions of the forces of cohesion. The short vertical lines downward from each molecule indicate the lines of the force of gravity and the arrowheads on the cohesion lines mark the opposing forces of heat and cohesion. In the triangle, C a d, for example, the hypothenuse C d represents the total force of cohesion; C a is its vertical component, and a d its horizontal component. The resultant of all these horizontal components ad, ac, ab, etc., is a force effecting a compression of the surface of the water. A good idea of the structure of surface compression is shown by the ripe seed-tuft of the common dandelion.
Oh, but water is not compressible. True enough, to any sensible degree by an exterior force; but the interior forces at work in water do many wonderful things. For instance, they cause water to expand on cooling and to contract on heating, between 0°C. and 4°C., and all the water phenomena of oceans, rivers, and rain-fall, of hydraulic and of steam powers, and of the irresistible force of freezing, are caused by the molecular activities existing in a drop of water.
One reason for lack of a clearer understanding of these phenomena is the failure to perceive the fact that the tendency to form a sphere of water in the tumbler is incessant, whether the attraction of gravitation acts on the mass of the water freely, as in falling; is warded off, as in Plateau’s experiment; or is super-imposed upon the attraction of cohesion, compelling the water to conform to the interior shape of the tumbler and rendering the ever-present cohesion inconspicuous.
The action of water from the higher degrees of temperature, through 4° C. to ice, is shown by the accompanying drawings. A mole¬
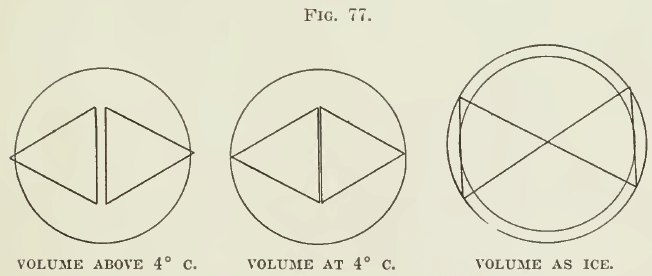
cule of water, composed of three atoms, is plausibly represented by a triangle. Two such molecules are separated a certain distance by a corresponding amount of heat, and this distance fixes the volume of the mass of water, which increases and diminishes as the degree of heat is raised or lowered. At 4° the volume of water is at a minimum and it is a fair inference that the molecules of water are at that point nearer to each other than at any point. Below 1°C. the molecular forces react in such a manner as to cause a change in the relations of the molecules themselves, causing them to turn and—in the state of ice—to assume the positions shown in the third figure, with a lower specific gravity than the water had before freezing. No other forces are necessary to the causation of the phenomena indicated than those of cohesion and heat.
Here, then, is the answer to the question asked at the meeting: By reason of the horizontal components of the attractions of cohesion which draw each molecule of water toward the centre of gravity of its mass, the surface of the water is compressed, made more dense, and offers a resistance to the needle greater than the weight of the needle. That weight is not sufficient to break apart the surface molecules, but only makes a slight indentation on the surface. When the needle is wetted, capillary attraction raises the compressed surface over and above the needle which, no longer resting upon the denser surface, but in water not under surface compression, obeys the attraction of gravitation and sinks.
Attention was called above to the two cases of simple compression where the entire surface of both liquids, the water in the brim-full tumbler and the mercury in the partly filled glass vessel, are convex, whereas in a tumbler partly filled with water, the edge of the water, where it meets the glass composing the tumbler, is concave and the water wets the glass. Thus there is added a new force which modifies the surface compression of the water and draws the water at the edge upward on the glass, forming a concavity tangent to both the surface of the water and the inside of the tumbler. It makes no difference here and now what this force is called, whether cohesion or adhesion: whether it is the same molecular attraction that exists between the molecules of the water or whether it is the cohesion of the glass acting at sensible distances, or neither, or both. The water is drawn up, not pushed up, and any drawing up is attraction, and acting on molecules it is molecular attraction.
In a tumbler 2½ inches in diameter the horizontal concavity against the glass seemed to be about ¹⁄¹6 of an inch wide, perhaps a little more, leaving about 2²⁄³ in. of convexity to ¹⁄8 in. total concavity, out of the diameter of 2½ in. The vertical concavity seemed also about ¹⁄¹6 of an inch along the inside of the glass. With glass tubes of smaller diameter the horizontal concavity seemed to remain about the same, but the vertical concavity increased as the diameter diminished. The convexity at the centre of the surface decreased with the diameter of the circle and in a tube of ¼ in. diameter the surface of the water was an inverted hollow sphere with no convexity at all and its height above the level of the water in the tumbler was 1/8 of an inch. “With a tube 1/16 of in. diameter the water came up 1/4 inch.
The surface compression at the edge of the water in the tumbler is less than nearer the centre, being practically zero, and offering no resistance to the upward attraction upon the water. If a glass tube be partly immersed in the water in the tumbler, the water in the tube, even if open at the lower end, forms a separate cohesive mass, independent of the rest of the liquid with all the phenomena of capillarity.
It has been said above that the cohesion of the water varies inversely as the temperature, being greater at the lower than at the higher temperatures, and at the boiling point there is no cohesion. With the same changes in temperature the attraction between the water and the glass sides of the tumbler varies exactly as the cohesion varies, and there is every reason to believe that the forces elevating the liquid are those of cohesion of the water and the glass acting at sensible distances. These phenomena between the water and the mercury on one hand and glass on the other are, of course, those of capillarity. They seem to fit in with the above theory of surface compression.
Then what is there left of true surface tension? Well, there is the soap-bubble. I made some experiments in this direction a few days ago with 50 or 60 soap-bubbles from 4 to 6½ inches in diameter. These were burst over a dark hardwood table about 30 inches square, so that the resulting wet spots on the surface of the table could be examined. Care was taken in every case in blowing the bubbles to remove the usual drop of water at the south pole of the bubble, so that all the wet spots came from the wreck of the distended film. After each bubble burst the table was wiped dry for the next one. When inflation ceased, one bubble 5 inches in diameter shrank an inch before it burst; another shrank from 6½ to 5 inches. In both cases the air was expelled by a real surface tension of the bubble’s film. Most of the bubbles were blown until they burst, at from 1 inch to 2 feet above the table. The ones at 1 inch spread wet spots in circles from 7 to 13 inches in diameter. Of the bubbles that burst at greater distances from the table, at 6 inches above the table the wet spots extended to the edge of a circle 15 inches in diameter; at 12 inches above, 20 inches; at 20 inches, 24 inches; and at 24 inches, 30 inches. Counting a quarter circle, there were from 175 to 260 wet spots, or 700 to 1000 for each bubble.
It was evident that the force throwing these drops of water such great distances was not the air pressure inside the bubble. When the bubble burst the attraction of cohesion of the water composing the film acted to re-unite the distended watery molecules and, as the shortest distance between two points on the circumference of a sphere is measured on the great circle that joins them, the re-uniting molecules took that route, traveling over the spherical surface of the bubble, and when a number of them met and formed a drop, all the molecules were attracted with a certain force. The tangential components of these cohesive forces, acting in the substances of the spherical film and at right angles to the bubbles’ radii, neutralized each other, while the centrifugal components united to shoot the drops away from the centre of the late bubble, in the direction of the prolongation of the bubbles’ radii, and they fell in a wide circle, as already stated.
This is a true statement of the phenomena of the effect of surface tension on a soap-bubble. By what stretch, by what torture, of the imagination can these phenomena be brought into identity or even the least resemblance with those of the placid floating of the greased needle upon the compressed surface of the water in the tumbler ?
Mr. Charles T. Durell in an article in the Mining and Scientific Press of September 18, 1915, entitled ‘Why Is Flotation?’, discusses the rising of a bubble through a liquid and says: “Surface tension causes the molecules of the liquid to form a film around the bubble and remain with it to the exclusion of like molecules during the time the bubble remains in the liquid. To all intents and purposes, this film is seen to be the same as if it were a membrane of some solid. The air in these bubbles can no more come in contact with the liquid through which it is passing than it could were it inside a toy balloon, for instance. The bubble may be said to be enclosed in a ‘liquid skin.’ As a proof of his argument he cites in a footnote the following: “A striking experiment to show these liquid films is as follows: To a breaker partly filled with a colorless oil, add a small quantity of permanganate solution. Blow air through a finely drawn-out glass tube into the permanganate solution now on the bottom of the beaker. Air bubbles enclosed in the colored liquid film rise through the oil and break at the surface, because of the expansive force of the gas. The colored water drops back through the oil exactly in the same manner that a balloon, bursting, drops to the earth.”
“With these instructions the following experiments were made: A layer of water, half an inch thick, colored dark blue with a dye not soluble in kerosene, was put into a tumbler and three inches of white kerosene poured upon it. With a medicine dropper having a rubber bulb and a 1/16-in. hole in the end of the glass tube, bubbles of air were blown into the blue water, the end of the glass rod resting on the bottom of the tumbler. At first the pressure on the bulb was made very gently, the idea being to have the bubbles as small as possible. As many as 200 of these tiny bubbles were blown and they rose to the surface and formed a group. Some burst, some were incorporated with others, and finally, of course, they all burst. Every one of these 200 bubbles burst within a circle of half an inch, and that circle from the time of the first bubble until the last one, was not free from bubbles, one touching another and all forming a single compact group; but at no time, in the strong sunlight, was there the slightest trace of blue in the circle nor anywhere in the kerosene. The upward bound bubbles were perfectly white and there were no return passengers. The bubbles had no films but were simply holes in the water. When they came to the joint surface of blue water and kerosene, they slipped into the kerosene, made holes in that, and burst at the surface with no trace of a film.
Then, with greater pressure on the bulb, larger bubbles were blown, and with them, small quantities of the blue water were forced up into the kerosene. When these came separately the air rose to the surface and the water dropped back, but where they came together the air buoyed the water up to the surface where the air escaped and the blue water sank through the kerosene and disappeared. With greater pressure the bubbles became still larger, as did also the size of the drops of water forced out with the air. Where trapped together the larger masses of air and blue water joined and rose to the surface, as a single entity, sometimes very rapidly and sometimes very slowly. But in no case, whatever the size of the constituent parts, was the air-bubble blue. There were never any water-films. The rising combined air and blue-water drops in the cases of the larger bubbles were in shape as if the bubble were sitting on a tiny blue feather bed. In every case the blue water was below and the white bubble above and the bubble was pulling the drop to the surface. Sometimes the drop was too heavy for the bubble to float and both sank to the water layer and remained stationary until the drop merged in the blue water and the bubble was released.
When the smaller bubbles rose to the surface of the kerosene they did not break as quickly as in water but seemed to strike against the under side of the surface stratum and rebound downward and moving over to the edge of the tumbler. On nearing the glass they seemed to rise as if attracted upwardly, like the part of the surface stratum around the edge under capillary attraction.
Some other interesting phenomena of capillarity were noticed. In the blue-drop-kerosene experiment the sides of the glass were wetted by the kerosene, even below the joint surface of the liquid; but notwithstanding this fact there was observed the concavity of the blue water under the oil, seemingly warranting the belief that the attractions between the water and the glass took place through the intermediate film of oil.
With a body of mercury, a glass tube pushed below the surface showed a rounded surface of mercury within the tube, with no capillarity, the rounded surface being due solely to surface compression. With the tube floating in the mercury the level of the outside mercury was exactly the same as the top of the rounded contents of the tube; but when the tube was pressed down into the mercury the level of the mercury in the tube was lowered. It seems likely that the indentation of the floating needle and the lowering of the level of mercury in the glass tube are both due to the resistance of the surface compression to the entrance of foreign bodies.
In the experiment of the blue water, the bubbles and the kerosene, we come most unexpectedly upon flotation, or its counterfeit. If it is flotation, like the mineral flotation, how is it to be accounted for? If it is different, what is the difference? Will an explanation of the blue-drop kerosene flotation be that of mineral flotation, or will it help in that direction ? There is surely an attraction between the air- bubble and the blue drop, or why should they stick together? The blue drop is heavier than the kerosene and the bubble of air lighter. One pulls up and the other pulls down. Why do they not separate unless there is a positive molecular attraction between them? Why does the bubble, resting upon the blue drop, buoy both to the surface of the kerosene, except for some molecular attraction between blue drop and bubble? Where this attraction is manifested, even slightly, it is helped by the static pressure of the liquid medium in which the flotation takes place.
The great unsolved problem in flotation is the identity of the forces that do the floating. Some say that it is surface tension, some electricity, and some molecular attraction between the air-bubbles and the metallic particles; and there is always the mystery as to exactly the part played by the oil. In this article it is intended to show that there are certain molecular attractions between widely different substances which would seem to be nothing more or less than the force of cohesion acting at sensible distances, but for the circumstance that such an interpretation runs counter to our pre-conceived opinions as to molecular attractions; but these attractions are shown in this article to exist between glass and oil, between glass and water, directly and through an intervening film of oil, between glass and air, and between water and air. The impression remains that a thorough examination of our pre-conceived opinions may show that they are fallacious.
There are strong reasons for believing that the state of science today is not unlike that of learning at the end of the 12th century, at the time of the great awakening, when the world dropped the scholasticism of Rome and went back to the philosophy of ancient Greece. We have lost the faculty of studying phenomena, we are ignorant of the first principles of logic, and we have degenerated into mere juggling with names.
Proof of this indictment is found in Vol. XXIV of the Encyclopedia Britannica, at page 401-2, where it is stated that the passage of electricity through liquids had been explained as a transference of a succession of electric charges carried by moving particles of matter or ‘ions.’ Then it was discovered that the moving particles that carried the electric current were much smaller than the atoms of hydrogen, and they were re-named ‘corpuscles.’ They enter into the structure of all matter. The only known properties of these corpuscles are their mass and their electric charge. There is reason to believe that the whole apparent mass is an effect of the electric charge. “The idea of a material particle thus disappears and the corpuscle becomes an isolated unit of electricity—an electron.” This is a typical ‘scientific explanation.’ It starts out inventing the word ‘ion’, which it immediately re-christens ‘corpuscle’ and then ‘electron,’ and the only meaning that can be extracted from the argument is that electricity is supposed to be made up of units, a purely gratuitous assumption. Here is another on the same page 402: “Maxwell and Hertz showed that the velocity of propagation of light and electro-magnetic waves was identical and that their other properties differed only in degree. Thus light becomes an electro-magnetic phenomenon. But light is started by some form of atomic vibration and to start an electro-magnetic wave requires a moving electric charge.” Here are three sentences all fallacious.
The peculiar tendency of the human mind which substitutes empty names for real phenomena and then plays with the names is the same that makes religious peoples worship idols instead of fixing their minds on principles. It is easier. A pilgrimage to a shrine where one may worship a rag, a bone, or a hank of hair, and be absolved, is less trouble than leading an exemplary life. So that when the question is asked “Why does a drop of water that falls upon dust take the form of a sphere?” it is easier to say “Oh, surface tension” and let it go at that than to think about it. It is all very well to say that a snark is a boojum, if you first define your boojum; but when you scratch the boojum and find the same old snark the pursuit of knowledge seems in vain.
