What is cyanide and why do we need cyanide-free gold leaching process?
Cyanide (CN–) has been used since 1889 to recover gold from hard rock. As sodium cyanide (NaCN), it reacts with gold, oxygen (O), and water (H2O) to form a gold cyanide complex (Na
[Au(CN)2] ) and sodium hydroxide (NaOH). The chemical reaction dubbed the “Elsner Equation”, named after its discoverer, is shown below:
4 Au + 8 NaCN + O2 + 2 H2O → 4 Na[Au(CN)2] + 4 NaOH
Cyanide can be manufactured, stored, transported, used, and disposed of in a safe manner, occurs naturally, is not toxic in all forms or all concentrations, does not persist in the environment, and does not accumulate. However, when not managed properly cyanide can be dangerous.
The Baia Mare cyanide spill is a prime example of mismanagement where a dam containing contaminated waters burst and spilled over some farmland and into the Someș River killing large numbers of fish. The accident has been called the worst environmental disaster in Europe since Chernobyl. Incidents like these have resulted in a ban on cyanide use in gold extraction by governments such as Germany, the Czech Republic, Hungary, and Turkey. Even some places in the United States have passed anti-cyanide legislation including Montana and Wisconsin.
Consequently, mining projects that would have been economical in areas where anti-cyanide legislation has been passed are no longer viable. Therefore, a cyanide-free leaching process is needed that can compete with the costs and recoveries associated with cyanidation while reducing environmental liabilities.
What are halogens and why are they used?
The halogen family of elements includes fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At) (Figure 1). These elements have seven electrons in their outer shell, and are one electron short from fulfilling the octet rule (8 electrons required for stability). The missing electron results in a strong affinity to react with other elements or compounds to acquire an 8th electron and achieve stability. The reactive characteristics of these elements are of particular interest to the mining processing industry as they can be used to extract precious metals such as gold (Au) and silver (Ag).
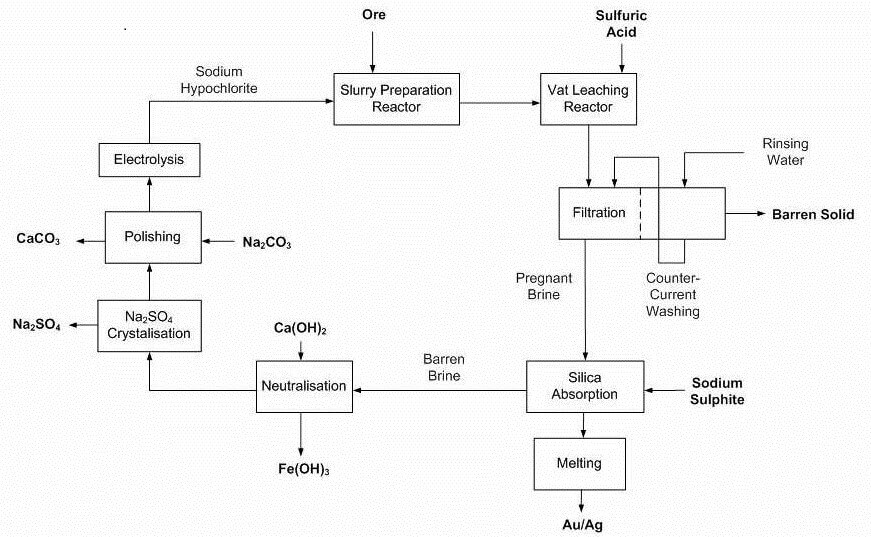
Advantages claimed by Dundee’s method
- Closed loop, doesn’t generate liquid waste requiring tailings ponds to be built
- Process/design flexibility can treat precious metal deposits containing base metals
- High gold recoveries achieved
- Tailings are inert from toxic substances, sulphides depleted and not acid generating
- The efficiency of the process, coupled with its operating conditions, plant size, construction material allow for competitive operating and capital costs as confirmed by technical and economic validation done by recognized engineering firms.
Sources
Infomine Cyanide
https://www.infomine.com/publications/docs/SummaryFactSheetCyanide.pdf
Cyanide Bans
https://www.huffingtonpost.com/adam-cernea-clark/is-cyanide-in-its-autumn-hours_b_3974634.html
River incident
https://news.bbc.co.uk/2/hi/europe/642880.stm
