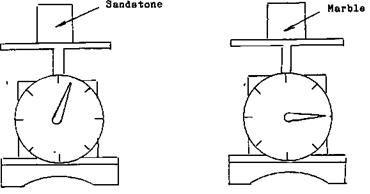
In the method we use to How to Find and Determine Specific Gravity of Rocks (some call it Specific Density because they don’t know better!), we’ll use simple equipment. What is required to make this measurement is a weight scale, and the list of the different minerals SPECIFIC GRAVITIES. What specific gravity is, is the mass of a solid in relationship to its weight. An extreme example of this is the difference in volume of a pound of feathers and a pound of gold. You could very easily put a pound of gold into your pocket and walk away with it but try and put a pound of feathers into your pocket. Your pocket just isn’t big enough. If you take a block of sandstone that is 2 inches by 2 inches and weigh it, then take a block of marble that is 2 inches by 2 inches and weigh it you will find that the marble far out weights the sand stone. This means, that although they occupy the same amount of space, their weight is definitely different.
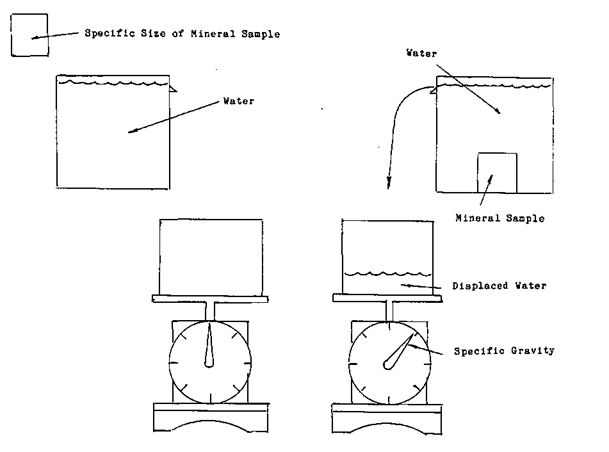
To determine each minerals specific gravity, a standard to measure each against was needed. The standard that was chosen was the weight of water.
To determine the specific gravity of a mineral, you simply take a sample of ore of known size and put it into a volume of water. Weigh the water that was displaced by the mineral. This will give you the ratio between the mineral and the water. An example is if a mineral that weighs one hundred grams displaces twenty grams of water the specific gravity of the mineral is 5*0 or the mineral weighs five times more than the same amount of water. This process should not be confused the rock tumblers you see being used in the hobbyist sector.
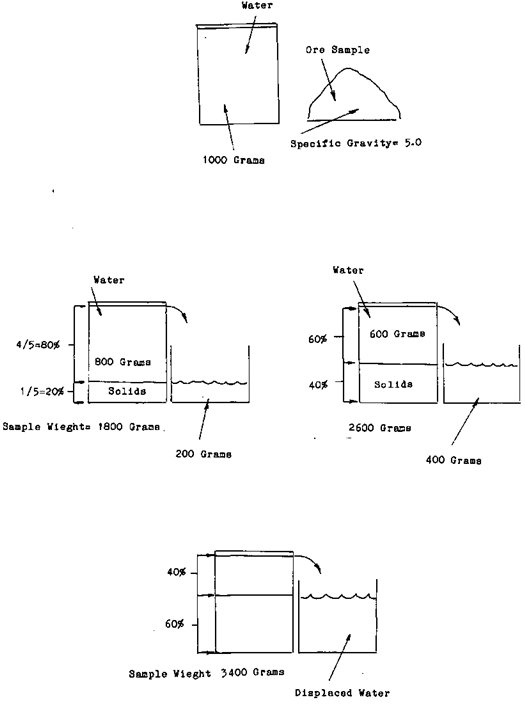
Once you know how much the mineral weights in relationship to the water the rest is easy. As an example One liter of water weights one thousand grams. If the specific gravity of a mineral is 5*0 or five times as much as the water, by taking a one liter sample of slurry and weighing it you can determine the percentage of ore that is in the one litre sample. As an example let us say that the weight of the sample was three thousand four hundred grams. You know that the ore weighs five times as much the amount of water that it displaces. In other words If a one thousand gram sample of ore was measured it would be one fifth the volume of the equal weight of water. Or for every time the weight of the sample grew by one thousand grams one fifth or twenty percent was ore. The sample weight grew by one thousand grams minus one fifth of the total weight of the water. This is the amount that the ore displaced. It weights one thousand grams (the weight of one litre of water) divided by five equals two hundred grams. This means that our thirty four hundred gram sample represents sixty percent solids, forty percent water.
How to Obtain the Specific Gravity of Dry Solids
To obtain the per cent solids in a pulp, from the specific gravity of that pulp, the specific gravity of the dry solids in the pulp must be known. The following is a simplified procedure for obtaining this figure.
Place a weighed amount, say 200 grams, of dry solids in a dry graduate. Add to this graduate a measured volume of water, say 500 cubic centimeters (or milliliters). Measure the increase in volume caused by the dry solids displacing some of the water. Assume the new volume is 550 c.c. The displacement, then, is 50 c.c. (550-500). Divide the weight of the dry solids (200 gr.) by the difference in volume (50 c.c.). The result, 4.00, is the specific gravity of the dry solids.
This video explains this specific gravity determination well and the Archimedes’ Principle.
https://www.911metallurgist.com/milling-calculations-formulas