Table of Contents
- Form
- Color
- Refractive Index
- Ring Members
- Crystal Structure
- Crystal Size
- Crystallinity
- Effective Pore Diameter
- Crystal Void Volume (cc/cc)
- Packing Density
- SiO2/Al2O3 Ratio
- MOH’s Hardness
- Moisture as Packaged
- pH of 1 % Dispersion
- Stability
- Ion Exchange Capacity
- Ammonia Exchange Capacity
- Typical Chemical Analyses
- Exchange Selectivities
- Exchange of Heavy Metal Ions
- Treatment of Mining and Metallurgical Effluents and Processing Wastes
- Conclusions
Most natural zeolites now being produced are used in low tech applications such as horse stall deodorizers, aquarium filters, and animal adsorbents.
Characterization of Natural Zeolites: Before any uses or applications of zeolites to the recovery of heavy metals from mining or metallurgical effluents can be seriously considered it is necessary to characterize the zeolite minerals. Characterization makes it possible to match the physical and chemical properties of the zeolite mineral to the environment of the application in which it must operate. This is not easy because the product data sheets provided by most natural zeolite producers usually contain only limited information on their product. This is in stark contract to synthetic zeolites whose producers not only have comprehensive product data sheets but field engineers and laboratory facilities to assist in tailoring the zeolite to the specific application. Only UOP and the Norton Company who are principally synthetic zeolite producers provide the product data and technical support for their natural zeolite products.
Characterization begins at the zeolite deposit. This means that a zeolite deposit must be thoroughly explored to determine its extent, continuity and quality. Both the mined and processed product must be carefully sampled to insure the purchaser a dependable supply of zeolites which are of a consistent quality. The specifications which are used for grade or quality control must match the specifications required for the intended use or application.

The characterization of zeolite minerals initially requires careful screening techniques to identify the zeolite and gangue minerals and to determine the percentage of each present in the product. Next the elemental composition of the product should be determined. Both the mineralogy and the elemental composition must be known before the zeolite can be considered for any ion exchange applications. If the zeolite product contains a high percentage of usable zeolite minerals usually chabazite, clinoptilolite or mordenite and does not contain significant quantities of the minerals smectite (montmorillonite), calcite or dolomite or the elements potassium, magnesium or calcium then the product should undergo additional testing.
The additional testing will determine other properties such as the ammonia exchange capacity, pH stability, exchange selectivities, and exchange capacities for selected heavy metal ions. These are some of the properties which must be matched to the specific application if the product is to perform successfully. In the past zeolite products have been introduced into effluent streams and expected to perform without any information on the composition of either the effluent stream or the zeolite product. Clearly this hit or miss approach has seldom ever been
Tables I and II are reproductions of zeolite product specification sheets which will be used to characterize this product for potential hydrometallurgical applications.
Using this example should in no way be construed as either an endorsement or recommendation of the product. If another zeolite is better suited for an application it will be mentioned. There are also comments on the data sheet and recommendations which are intended to make it more useful.
Form
This product is available in powder and granules. The powder can be used in mixing tanks and then thickened or filtered out of the solution or mixed with sludges. The granules are suitable for use in filtration columns or launders.
Color
The product is a-light brown color and has a dry brightness of 43. Color is not usually important for commercial ion exchange applications. It is important for consumer applications such as ammonia removal filtration columns made of transparent plastic which are used in aquariums.
Refractive Index
This is not important in ion exchange or any other applications. It could be omitted from the product data sheet.
Ring Members
Chabazite has a framework with large ellipsoidal cavities. The entrance to these cavities is through six, 8 ring pores. This array produces cavities 11 by 6.6 Angstroms which are joined to adjacent cavities through six, 8 membered rings having free diameters of 4.1 by 3.7 Angstroms. Chabazite is of the most porous natural zeolite having a surface area of 500 to 600 square meters per gram. This information would have been more useful if the surface area which affects reaction time had been either used with or in place of the eight ring members.
Crystal Structure
The crystal structure should be omitted from the data sheet because the structure can be better defined by framework topology which is designated by the Structure Commission of the International Zeolite Association.
Crystal Size
This is an estimate of the size range of the individual chabazite crystals. Actually most of the crystals are in the two micron range, consequently, it would be more accurate to state that 80% of the crystals are less than 5 microns in size. Such a fine particle size indicates that reaction times will be very rapid.

Crystallinity
It is unusual for a natural zeolite to have a crystallinity of over 90%. This indicates that 90% of the product is zeolite with the remainder being non zeolitic components, principally unaltered glass.
Effective Pore Diameter
This is a measurement of the minimum diameter of the six, 8 ring pores. These pores are somewhat larger than those in chabazite from other deposits. The size of the pores determines the maximum critical diameter of a molecule which can enter the pore. This means that water which has critical a diameter of 3.2 Angstroms can be separated from ethanol which has a critical diameter of 4.4 Angstroms. This property is important for gas adsorption and not for ion exchange applications.
Crystal Void Volume (cc/cc)
This measurement means that 47% of the dehydrated zeolite is open space. At ambient temperature and humidity the open space is filled with water. For ion exchange applications this means much greater access to active exchange sites.
Packing Density
The packing density determines the weight of the powder or granules that can be packed into a cubic foot or cubic meter. This weight is dependent upon the temperature and humidity. It is also dependent upon the particle size distribution. Most purchasers of cation exchange media purchase the product by volume (cubic feet or cubic meters) rather than by weight (pounds or kilograms). The purchaser should be made aware that the packing density can be misleading.
SiO2/Al2O3 Ratio
The silica to alumina ratio is very important in ion exchange applications. The lower the silica to alumina ratio the higher the ion exchange capacity. Conversely a high silica to alumina ratio generally means a higher the stability in low pH solutions. Chabazite begins to dealuminate at a pH of under 4. Clinoptilolite is stable to a pH of 3 and mordenite to a pH of 0.6 – 0.75. Although clinoptilolite and mordenite do not effectively extract copper under these highly acidic conditions, erionite another zeolite, will extract modest amounts of copper even at a pH of 2. This means that erionite can remove copper from acidic solutions which would cause chabazite to begin to dealuminate.
MOH’s Hardness
This is an antiquated method of determining hardness. The Vickers hardness numbers are much more accurate. However, an abrasion test such as ASTM Method C 131 or C 535 used for aggregate would be far more useful. In ion exchange applications particle attrition caused by purging a bed can cause filters to plug with fines if the media is not attrition resistant. Chabazite because of its high crystal void volume is less resistant to attrition than either clinoptilolite or mordenite.
Moisture as Packaged
This is a measure of the weight of water which is held in the pores as packaged.
pH of 1 % Dispersion
A pH of 10 is a relatively high pH. However, a pH higher than 10 strongly suggests contamination with calcium carbonate.
Stability
This means that the chabazite will not dealuminate within this pH range. Clinoptilolite is stable to a pH of 3 and mordenite to a pH of 0.6 – 0.75.
Ion Exchange Capacity
This is the sum of the cations exchanged out of the zeolite in meq/g. This analytical technique determines how much of each cation was exchanged. This is important because if significant amounts of potassium, magnesium, and calcium are exchanged out of the zeolite then it is unlikely the zeolite can be used in ion exchange applications without being sodium modified. Many clinoptilolites contain potassium which is very difficult to exchange out of the zeolites. A high potassium zeolite does not perform well in ion exchange applications unless it can be sodium modified.
Ammonia Exchange Capacity
This is a measurement of the quantity of ammonia exchanged into the zeolite expressed in meq/g. From the consumers point of view it might be better to express the ammonia exchange capacity in weight percent ammonia retained after ion exchange.
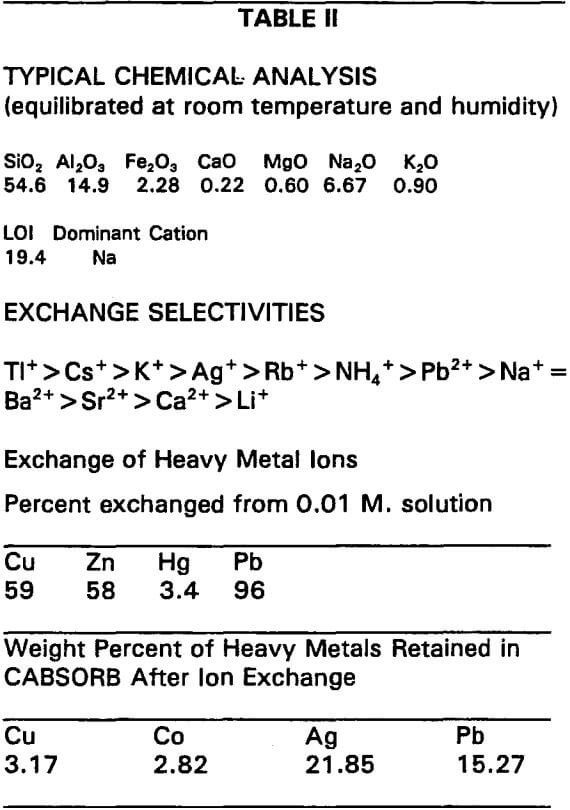
Typical Chemical Analyses
This would be more useful if the mineralogy of the product had been listed. This zeolite product contains about 80% chabazite 15% clinoptilolite and traces of analcime and erionite.
Exchange Selectivities
In a very general way the exchange selectivities are probably correct. However, based on our experience chabazite has a much higher selectivity for lead than for ammonia. It is important to understand that selectivities can change with changes in pH.
Exchange of Heavy Metal Ions
This is also correct in a general way. But changing the pH can dramatically alter the results. The weight percent of heavy metals retained is an ideal case where a single cation was exchanged from a nitrate or sulfate solution. Changes in pH and the addition of other cations could significantly alter these results.
In summary the product data sheets for natural zeolites need improvement. More data must be provided on the effect of pH on the removal of metals from solution. And in addition more research needs to be done on the removal of metals from waste streams when blocking cations particularly calcium are present and when several metals are in the solution which is usually the situation with mine and metallurgical waste streams.
Natural zeolite producers need to develop improved products which can successfully treat a broad spectrum of mine and metallurgical effluents. Some progress is being made. UOP, Norton, and Davison Chemical have produced activated extrudates for many years. The extrudates are uniform in size which means that the permeability of the bed is always constant. The extrudates are also attrition resistant which means that fines will not block off the flow. Waterman and Aplan were successful in making highly attrition resistant pellets from natural zeolites using several different types of binders. Pelletized zeolites which are being used in the removal of lead from effluent streams are now commercially available.
Research is also being done on treating waters contaminated with heavy metals using zeolites which have been modified with high molecular weight organic cations. The treatment significantly increases the amount of chromate the zeolite will take up. Clearly, improving the physical properties of natural zeolites by pelletizing or extruding them to increase flow rates and attrition resistance, sodium modifying them to increase their cation exchange, and developing surface modified zeolites for the removal of chromate and organics from waste streams should increase their applications in cleaning up mining and metallurgical effluent streams.

Treatment of Mining and Metallurgical Effluents and Processing Wastes
Table III shows the processing wastes which the EPA considers to be hazardous. Zeolites can be used to recover or stabilize these wastes for disposal. Each of these wastes has to be carefully evaluated and then matched to a specific zeolite product which has been characterized.
Sodium modified clinoptilolite, mordenite, or erionite granules might be suitable for the removal of heavy metals from low pH waste streams. Granules of sodium modified chabazite with its higher exchange capacity would be recommended if the pH of the solution could be adjusted to between 4 and 5. Zeolite powders can be used to stabilize sludges. And, the zeolites modified with high molecular weight organic cations could be used to treat sludges and liquids from ferro-chromium production.
Heavy metals can be either safely disposed of or recovered in several different ways. These include:
- Storage in a mine or hazardous waste facility. The heavy metals loaded on the zeolites reside in exchange sites within the crystal lattice and cannot be released into the environment.
- Fusion or vitrification into glass. Radioactive isotopes are usually processed using this technique. The chabazite product used to recover the radioactive isotopes at the Three Mile Island reactor was vitrified in an induction furnace, encased in concrete, and placed in underground storage at Hanford, Washington.
- Smelting. The valuable metals can be recovered by adding the zeolite loaded with copper, lead, or silver to the concentrates fed into the smelting furnace or converter. Zeolites fuse into glass at 593°C (1100°F).
- Hydrometallurgy. The valuable metals can be recovered by elution of the zeolite bed with a high pH sodium chloride solution. The metals can then be recovered in an electrolytic cell.
Conclusions
- Zeolites must be characterized to match them with the physical and chemical characteristics of a mine or metallurgical effluent or sludge.
- Zeolites provide the option of either stabilizing the waste products for disposal vitrifying them, or recovering the metals by smelting or elution and electrowinning.
- Zeolite products are becoming available in pellets and extrudates which resist attrition and greatly improve the flow rate of filtration columns. Sodium modified products have significantly improved cation exchange capacities. Zeolites which have been modified with high molecular weight organic cations increase their sorption of chromate, benzene, toluene, xylene from aqueous solutions.

