Table of Contents
The term “clay” is applied usually to certain earthy rocks whose most prominent property is that of plasticity when wet. This permits them to be molded into almost any shape, which they retain when dry. Furthermore, they harden under fire. Clays contain hydrous aluminum silicates—the clay minerals—in appreciable amounts, but aside from this a number of other mineral grains, particularly quartz, may be present. Texturally clays are fine grained, and the so-called true clay particles are usually under 2µ in diameter.
Minerals in Clay
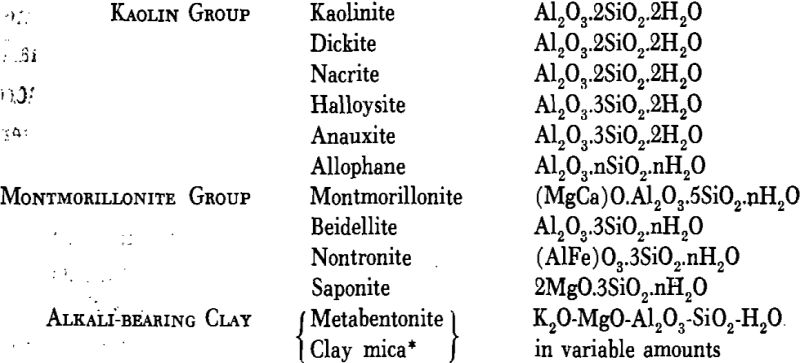
Certain hydrous aluminum silicates that predominate in many clays are known as the clay minerals and are grouped by Kerr as follows:
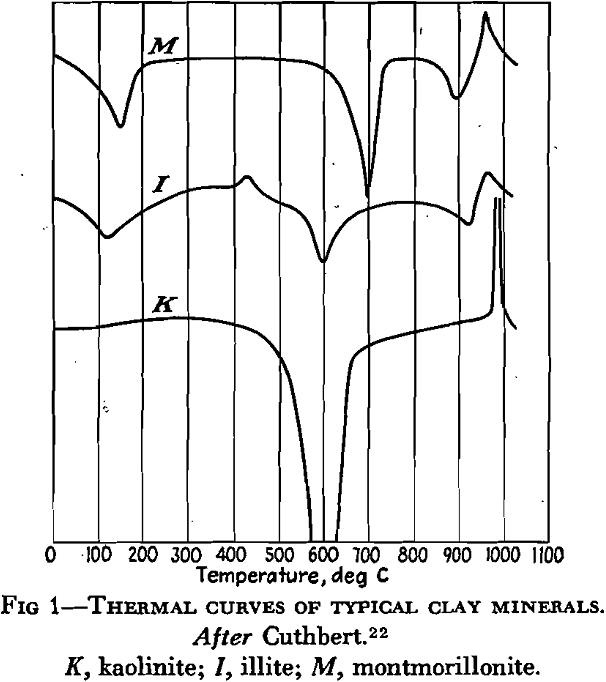
Many other minerals have been identified in clays but few of them occur in quantity. Those that may be present, sometimes in appreciable amounts, are: quartz, usually in grains of variable size; calcite, usually in fine-grained, perhaps colloidal form, but sometimes as concretions; limonite, often finely distributed as a coating on grains, sometimes as concretions or crusts; gypsum, in grains, selenite plates, crystals or rosettes; siderite, sometimes finely distributed, or occasionally as concretions in some clays and shales; pyrite as grains and concretionary lumps; muscovite, widely distributed, and commonly in very small flakes; rutile, almost universally present but only in scattered grains of microscopic size.
Clays do not all respond in the same manner to acids or alkalies, because each one may show its own acidity or alkalinity. Furthermore, the presence of soluble salts may exert a modifying effect. Dispersing electrolytes include sodium silicate, sodium hydroxide, sodium carbonate, sodium oxalate, sodium phosphate, and others. Coagulants include acids, sodium chloride, calcium chloride, aluminum chloride, and so forth. Some electrolytes, like sodium carbonate, may cause deflocculation when added to the clay in small amounts, and coagulation when larger amounts are added.
Base Exchange
The process of base exchange, representing the alteration in cation composition of a solid when treated with a salt solution, is probably of much importance in clays. According to the definition, base exchange may operate with either colloidal particles or larger grains of mineral matter; in other words, it may operate in the weathering of rocks or in fine-grained sediments. Opinions seem to differ as to whether base exchange involves a change in crystal structure. It is probable, however, that it may go on in clays either during or after deposition, and Ross and Kerr assert that it may take place without any breakdown of the clay molecule as a whole.
Properties of Clay
Plasticity
The property of plasticity, already defined, is the outstanding characteristic of clays. They vary from those of high plasticity, or “fat” ones, like the ball clays and bonding clays, to those of low plasticity, termed “lean,” and represented by some very sandy ones. The plasticity may be affected by the amount and character of colloidal material, the quantity and proportions of nonplastic particles, the amount of water, as well as salts, bases, acids and organic matter.
The cause of plasticity has been much discussed and has been variously assigned to hydrous aluminum silicates, shape and size of grains, colloidal content, and other attributes. The present general view regarding plasticity is well expressed by Norton, who says: “It is undoubtedly due to an active particle surface, which has the property of attracting to it a stable water film. This attractive force both holds the water in the pores and the particles together.” There is no doubt also that the plasticity is influenced by the thickness and viscosity of the water films around the particles, as well as the size, shape and distribution of the latter.
Texture
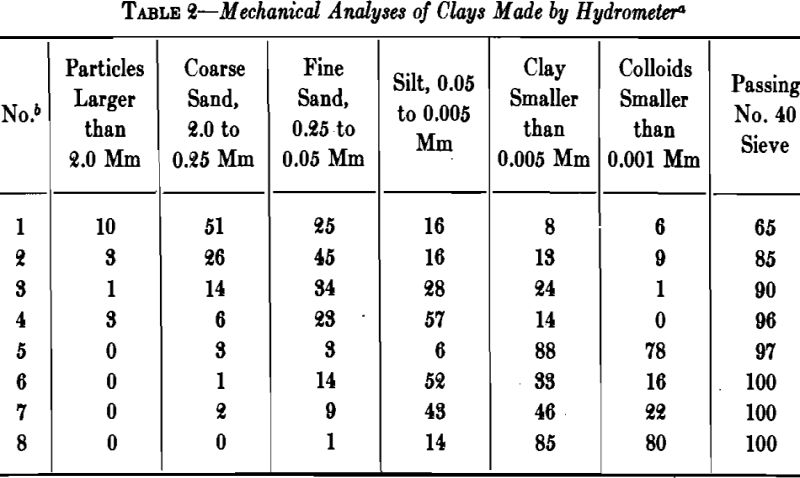
The grains of clay cover a relatively wide range of size, some clays containing sand particles large enough to be recognizable with the naked eye. A large percentage of .the particles may be less than 2µ (0.002 mm) in size, and when the clay is thoroughly dispersed the smallest, which are negatively charged, may remain in suspension for days.
Water in Clay
Two kinds of water usually are recognized in clay: (1) mechanically held water, and (2) chemically combined water. When a clay dries from its plastic condition to a constant weight at room temperature, the water that evaporates until air shrinkage ceases is known as “shrinkage water.” That which is still left in the intergrain spaces is termed “pore water,” and may be driven off at 100°C. The pore water and shrinkage water together are known as the “water of plasticity.”
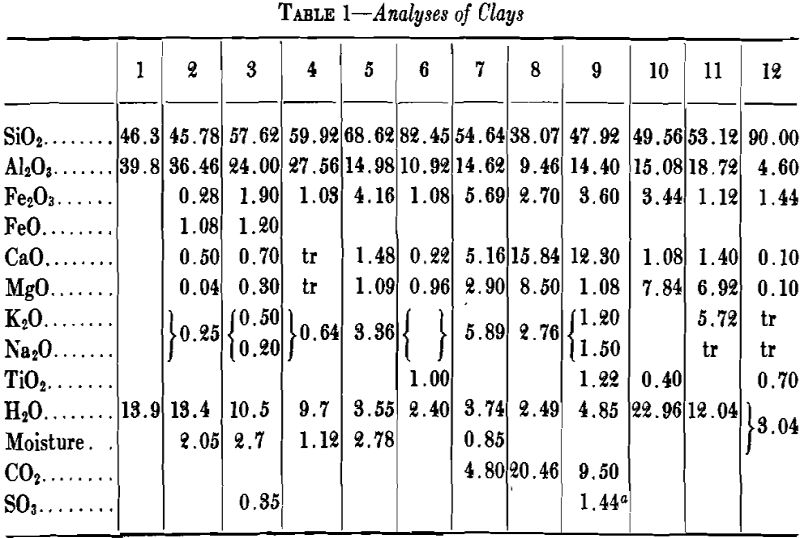
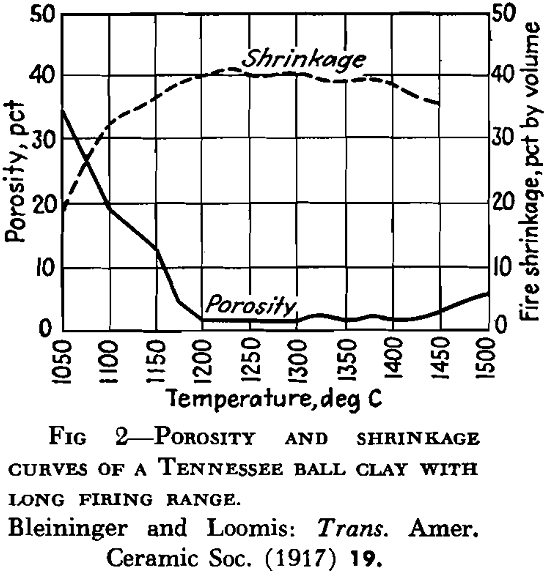
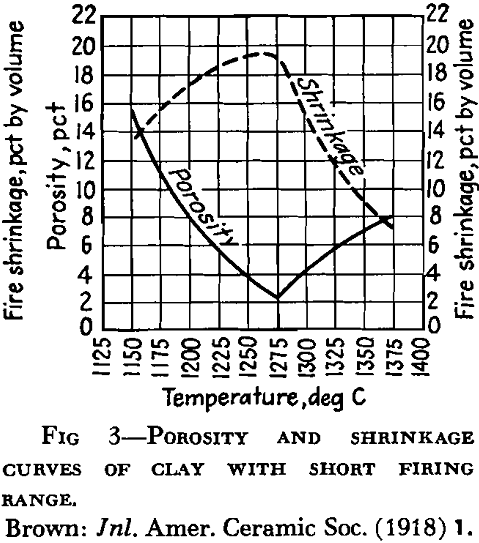
A high percentage of shrinkage water is rather characteristic of fine-grained clays that dry to a strong body and they are likely to show excessive plasticity, high shrinkage, warping and cracking. A high content of pore water characterizes a clay with a porous structure. The ratio of shrinkage water to pore water is said to be important in clays used in the manufacture of crucibles and glass pots and the best ones are said to show a ratio of 1:1 between shrinkage water and pore water. Table 5 gives the properties of several clays with respect to water and shrinkage.
Color
Iron is the commonest coloring agent of raw clays, giving yellow, pink, reds and browns, depending on the amount present and the state of oxidation. Greensand usually gives a green color. Organic matter may color a clay gray or black, sometimes even pink. Clays free from these coloring agents are usually white. Fired clays may owe their color to iron compounds, titanium oxide, or lime reacting with iron, but iron is the usual cause of the color.
Fusibility
When exposed to a rising temperature, clays do not fuse suddenly; on the contrary, they soften slowly until the entire clay becomes a viscous mass. With impure clays this may occur at a relatively low temperature but with those approaching kaolinite in composition it takes place at a much higher one.
Following dehydration, the clay is porous but after a temperature interval it begins to compact, then the more easily fusible minerals begin to melt, with the formation of glass. With further temperature rise the fluid portion attacks the mineral grains not yet fused, and finally a solution of molten glass is formed.
In the first stages of firing, although the clay may become compacted into a hard mass like common brick, it is said by some that there is no evidence of softening of the particles. This period has also been called incipient vitrification, but by some that term is used to refer to the stage at which enough glass has been developed to bind the mass together. Complete vitrification would represent the stage reached in the fusion of a clay when sufficient glass has been developed to close all the pores. As the temperature rises and the fluidity of the glass developed increases, the clay mass or object no longer holds its shape, and reaches a condition referred to as viscosity. The temperature at which the development of glass begins, as well as its amount and viscosity, exerts an influence on the behavior of the clay during vitrification. In some clays, glass has been found to develop at a temperature as low as 700°C. The term “vitrification” as applied to clay wares does not al¬ways mean the same thing. Paving brick and sewer pipe, for example, are said to be vitrified, but technical difficulties prevent the complete attainment of that condition. Electrical porcelain must approach it very closely.
Origin of Clay
Clays may originate from different kinds of rocks, either by the ordinary processes of surface weathering or by the action of solutions, which may be of igneous origin or indirectly of surface origin. In both cases the alternation product is of residual character and the material may be called a residual clay.
The removal of the clays so formed by various agents of erosion and transportation and deposition elsewhere gives rise to a great group of transported clays.
Residual clays due to weathering may be formed: (1) by the decomposition of the silicate minerals in rock, the product being a clay; (2) by the solution of a carbonate rock containing clayey impurities, which are left behind as an insoluble residue, and (3) by the disintegration, accompanied occasionally by some solution, of shales.
Distribution of Clays and Shales
Clays and shales are widely distributed in the United States and Canada, in formations ranging from pre-Cambrian to Recent. The more important types are:
Under the head of fire clays, we may include all clays that have a fusion point of cone 194 or higher. Certain varieties that are white- firing or nearly so are referred to often under separate names, as kaolin and ball clay. Excluding these, most of the fire clays that are of sedimentary character are used for a variety of purposes.
The ordinary sedimentary fire clays are found in the United States in formations ranging from Carboniferous to Tertiary, exclusive of the Triassic. Their geologic distribution in Canada is restricted to Cretaceous and Tertiary.
Foremost among the clays of this group are those found in the Carboniferous of Pennsylvania, Ohio Kentucky, Missouri, Indiana, Illinois, and Maryland. The clays occur at a number of different horizons, and may be associated with coal. Most of the material is plastic fire clay but with it there may be a hard type known as flint clay, which develops little plasticity and which, because of its texture, structure and appearance, has received this special name. It is found particularly in Pennsylvania, Ohio, Maryland, and Kentucky. Many of these fire-clay deposits are worked as open pits but others are reached by drift or shaft. These clays are widely used for firebrick and other refractory wares.
In their washed condition, kaolins are used largely in the manufacture of high-grade products, such as white earthenware, porcelain of all kinds as well as fillers for paper and rubber, and other products. They are rather limited in their distribution.
Bentonite is a sedimentary clay in which the mineral montmorillonite is an important constituent. It has high plasticity and bonding power. It is widely used as a bonding agent in synthetic foundry sands, in drilling muds, and for other purposes. Bentonite is a widely distributed material, and important deposits of it are found in Wyoming South Dakota, Mississippi, Arizona and Texas. It is also worked in western Canada, and deposits are known in a number of other countries.
Political and Commercial Control, Production and Consumption
It can hardly be said that control is much of a problem in the clay industry, because the United States is relatively independent of other countries for its supplies of raw clays. Only certain special kinds of high-grade clays have been imported and these have been gradually replaced by domestic materials, except perhaps the highest grade of paper-coating clays.
For many years there has been a somewhat steady importation of English kaolin or china clay for use in the manufacture of china, paper, paint, etc. The strong hold this product has had on the American market for years has been due in part probably to its more uniform character. Before the war more than two thirds of the china clay consumed in the United States was imported from England, and as late as 1925, according to Bureau of Mines statistics, the imports were more than the domestic production. The replacement of foreign clays in the pottery and paper industry continued during the depression, and in 1934 the china-clay imports formed less than 20 pct of the domestic consumption. This increase in the use of domestic materials has been due in part to their more careful preparation for the market.
Prospecting
A knowledge of the geological characteristics of clay deposits is of considerable aid in prospecting for clays and in making a field examination. All natural exposures as well as artificial cuts should be carefully examined, not losing sight of the fact, however, that on slopes or steep surfaces there may be considerable weathering and sliding of the material, which covers up a portion of the material in place.
In unconsolidated deposits, a carpenter’s auger attached to a sufficiently strong pipe may be used for boring the deposit. Such an outfit can be used to depths of 40 or 50 ft but the system should be made in short sections, which can be connected by means of coupling. An auger outfit can be used also for horizontal boring. The data obtained from properly located boreholes can be used to determine both vertical and horizontal variations in the deposit.
Mining
Most clay deposits are worked as open pits, the method of excavation depending on the character of the clay, thickness and extent of beds, and character of the overburden. Where the deposit is small, or consists of beds of different quality, which it is desirable to separate, the clay may be dug by spades or mattocks. Selective mining of different beds is practiced in New Jersey, western Kentucky and Tennessee, and also in part of California. Linton states that in some California deposits more than 20 ft thick, beds as thin as 2 ft may be separated in selective mining. With thicker beds, and a working face not more than 20 ft high, a power shovel may be employed.
At some clay pits the face of clay, if not too high, may be undercut at the base while wedges are driven in at the top, thus causing a slice of the bank to fall and break up the clay, so that it can be more readily handled. If the clay deposit has a horizontal surface of some extent, it may be loosened by plows and the clay then gathered up by wheel or drag scrapers.
Plasticity, Fineness, and Water of Plasticity
There is no standard test for determining the plasticity of a clay, although several different ones have been suggested. It is often determined by feel.
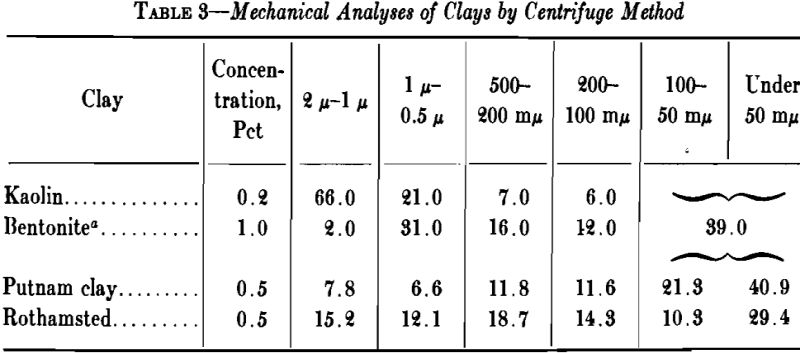
The fineness of clay may be tested by washing the sample through sieves and determining the weight remaining on each. Sieves are not fine enough to separate the smaller particles of clay, and for this purpose the hydrometer method, as used successfully by the U. S. Bureau of Public Roads, can be employed. Similar results are obtainable by the pipette method. The ordinary elutriator is not to be recommended, even though it has been much used in Europe.
Marketing and Uses
Clays may be sold under some name or number or under the name of the use to which they are put. This might mean that one clay might be sold under two different names.
Producers of the cheaper types of clay products, such as building brick, drain tile and even common red earthenware, with hardly any exceptions, obtain their clay from their own deposits. Only the kaolins, slip clays, paper clays, many refractory clays, bentonite and fuller’s earth (see chapters on bentonite and bleaching clay), as a rule, are mined by separate companies or individuals, and sold to consumers by the ton.
Clays may be used in their unfired or fired condition. The properties that govern the use of clays in their raw condition are color, texture, bonding strength and absorptive qualities. The uses to which unfired clays are put include:
Paper clays, usually kaolins of residual or sedimentary character. Whiteness, fineness and uniformity of texture are of prime importance. Somewhat similar clays are used as fillers in fabrics and in manufacture of ultramarine. This group may be designated as “fillers.” The requisite properties of fillers as specified by different consumers, either as groups or individuals, are somewhat conflicting, but whiteness and freedom from grit seem to be the essentials.
Bentonite, aside from its use as a bleaching agent, is used as a drilling mud in petroleum industry and as a bonding agent in synthetic foundry sands, which represent two important uses. It is also employed for stopping leakage in soils, dams and rock fractures underground, as well as in the manufacture of plastics.