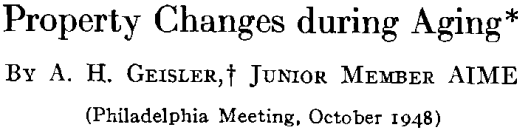The correlation of property changes during precipitation with structure has progressed, sometimes rapidly but other times more slowly, since the fundamental discovery of Merica, Waltenberg and Scott. The predominant factor that hampered the progress has been limitations imposed by the various detection methods. Refinements in X ray diffraction techniques and in metallographic methods, which have been extended by the electron microscope, have contributed new information within the last few years. These permit an extension of the theory as proposed by Mehl and Jetter in 1940. Now that a sufficient number of alloys has been investigated to establish a general mechanism of the precipitation process, a correlation of the facts into a generally applicable theory of age-hardening is both warranted and desirable. The purpose of the paper is to provide an orderly correlation of the phenomenological facts of precipitation hardening. Speculation on mechanism of hardening will be confined to that consistent with existing theories of hardening.
Age-hardening accompanies the formation of a new structure, the precipitate, as provided by an alloy system in which solid solubility decreases with decreasing temperature. Upon this premise alone all such systems would be expected to include age-hardenable alloys. Alas, this is not always the case. Few attempts have been made to explain the variable degree of age-hardening among different alloys or to explain the influence of small alloying additions upon the hardening. Dispersion hardening as originally postulated is not adequate. Coherency hardening—the term that will be used to distinguish the properties of a two-phase mixture in the unique and unstable transition state at which the phases are strained into precise atomic registry (continuity or coherency) in planes parallel to the interface—alone does not explain the course of the aging curves, but only the initial portion. In the present work, the principle postulated by Fink and Smith to explain double aging peaks is applied to the aging reaction as a whole. Brief mention has been made previously of the concept that the aging curve represents a summation of various hardening and softening processes. A further elaboration follows.
The Sequence
A general theory of age-hardening is facilitated by a general sequence of structure changes. The sequence for aluminum- silver alloys is proposed as the prototype for all precipitation alloys. Variations from this are only a matter of degree. The important consequence of this general sequence is that it reconciles the usual precipitation process with “discontinuous or heterogeneous precipitation.” Prior to the research on aluminum-silver alloys, the gap between the two was a source of confusion and prevented any common theory of age-hardening. Now ” discontinuous precipitation” is recognized as a subsequent reaction which follows continuous precipitation at any particular microscopic area. While there are yet certain intriguing features of the process, sufficient data exist to conclude that this is a recrystallization reaction. Thus, it will not be referred to as precipitation but as recrystallization or the “grain boundary reaction,” since the process is nucleated predominately at boundaries of the original grains much like the formation of pearlite from austenite in steel.
A general crystallographic sequence is desirable. That proposed involves simply a coherent transition structure prior to the formation of the equilibrium phase according to the reaction: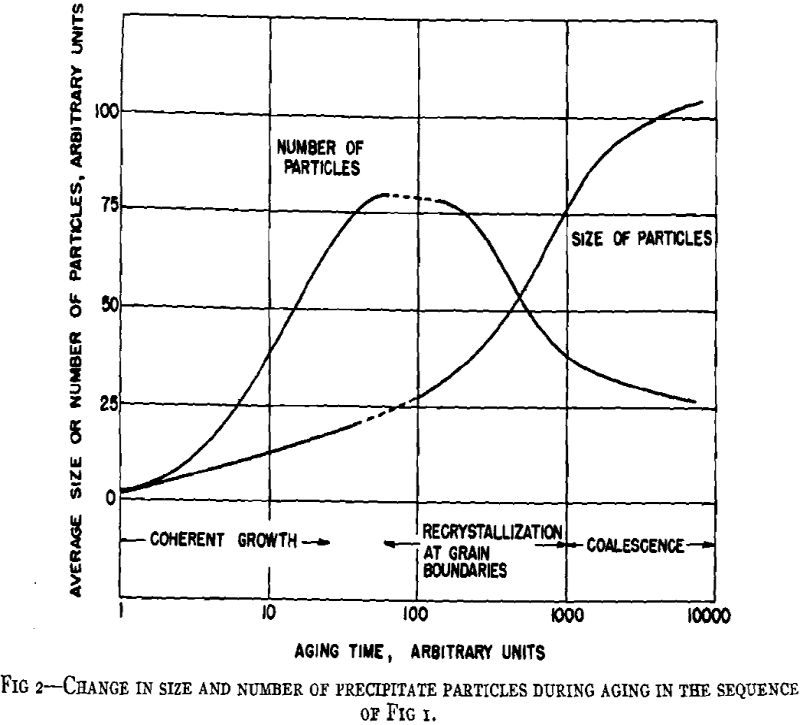
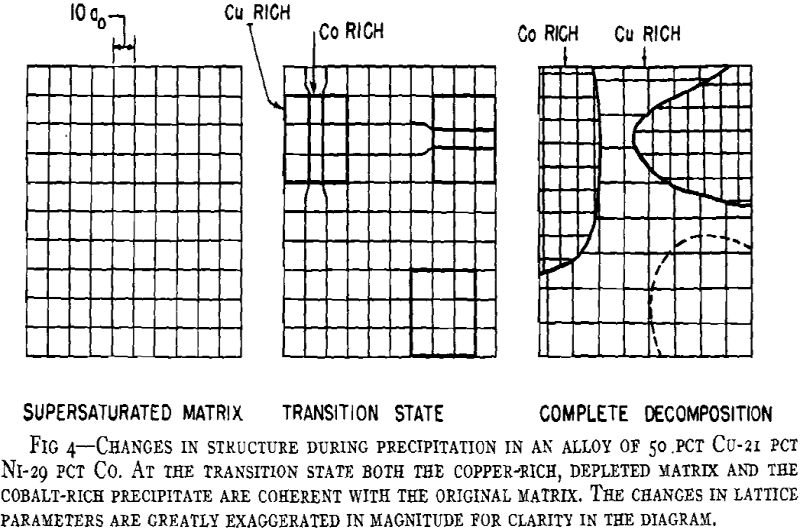
The Contributing Reactions
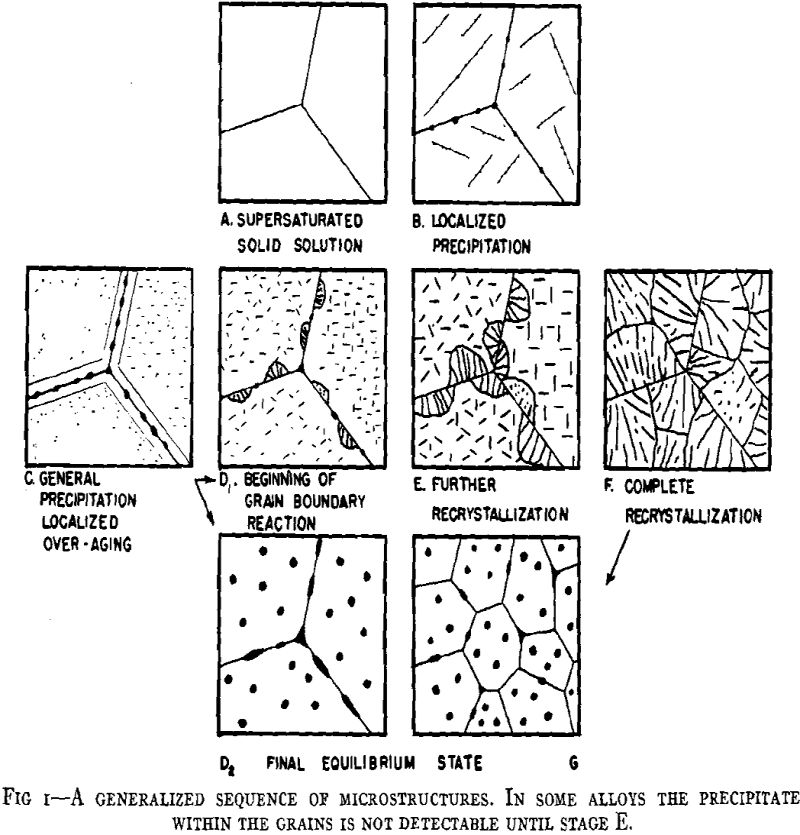
From considerations of the microstructural and crystallographic sequences during aging, the simultaneous occurrence of several reactions is apparent. Overaging at grain boundaries through loss of coherency of the precipitate, gross depletion of the matrix solid solution or recrystallization may be occurring at the same time as hardening within the grains through the formation of a coherent precipitate. In order to interpret the aging curves (property vs. isothermal aging time), these reactions should be considered individually. First, some general features of the initial process of coherent growth will be reviewed. The extent of coherent growth has been correlated with crystallographic disregistry between the second phase and parent matrix in the case of Al-Ag and Al-Zn alloys. With greater subsequent disregistry, breaking away of the precipitate occurs at a smaller particle size.
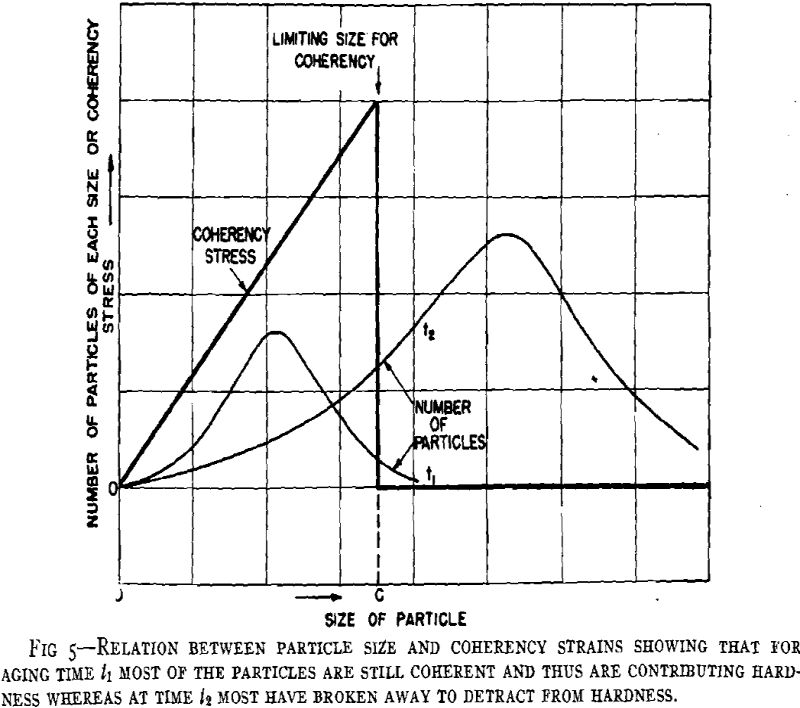
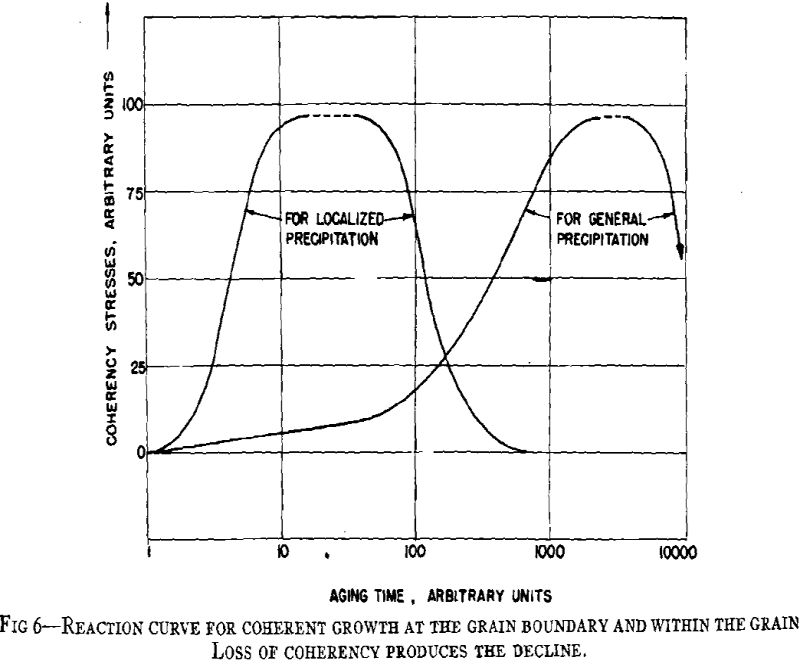
Before considering the sources of softening, other hardening processes should be evaluated. Dispersion hardening provided by the equilibrium phase may contribute slightly, but it is considered a minor reaction compared with coherency hardening for in no case has the equilibrium phase been found associated with pronounced precipitation hardening. This type of hardening has been based upon the dispersion of a hard phase in a soft matrix. A comprehensive study has been made in regard to the decomposition products of austenite in steel. Such hardening as originally defined is not applicable to precipitation hardening, for hardening may occur when both phases are soft (Al-Zn, Cu-Ag), while pronounced differences may occur in comparable alloy systems.
The property changes associated with softening reactions such as solid solution depletion, recrystallization or recovery and coalescence are well known.
Properties or the Coherent State
The physical and mechanical properties associated with the coherent state (Table I) are considered to be characteristic of this state in much the same manner as certain characteristics are attributed to the solid solution, the strain-hardened state and the ordered state. On this basis, the characteristic changes in properties such as electrical resistance, lattice parameter or specific volume are no more ” anomalous” than the increase in hardness. All are different from the expected change if the properties are considered to change continuously and directly from those of the supersaturated solid solution to those of the strain-free two-phase mixture. A review of the literature on age-hardening reveals that there are certain consistent changes which can be attributed to the formation of a coherent precipitate. These are summarized in Table I relative to the properties of the final equilibrium two-phase mixture. The magnitude of the changes depend on particle size only in so far as it determines the coherency strains in the matrix. The changes have been isolated for the coherent state of precipitation only. Other diffusion-type solid state reactions present broad fields for future research.
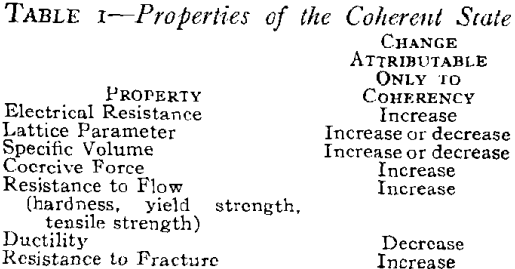
These are the characteristics of either the precipitate or the matrix when the two are coherent relative to their properties when they are not coherent. Some properties depend only upon the coherency strains in the matrix while others depend only on those in the precipitate.
The Property changes during Aging
The hypothesis relates the overall property change-revealed by the aging curve to an integration of the several hardening or softening reactions.
Electrical Resistance
At high aging temperatures when gross depletion of the solid solution occurs, the electrical resistance curve closely follows the change in average composition of the matrix solid solution. At low aging temperatures the resistance increase characteristic of the coherent state predominates. Here the depletion of matrix solid solution and the attendant decrease in resistance are slight.
Lattice Parameter
When gross depletion of the solid solution matrix occurs, measurements of lattice parameter provide a means of following the composition change and of revealing the heterogeneity of the process. However, the measurements are not sensitive to localized changes so that in the absence of a change the only permitted conclusion is that the solid solution depletion is slight. Indeed, it should be only slight for maximum hardening according to the proposed theory.
Resistance to Flow
To have practical utility a theory of age-hardening must be capable of explaining changes in mechanical properties to such a degree that predictions can be made for future alloy development. While these properties have been extensively investigated, there has been a certain reluctance to attempt specific explanations of any property other than hardness. With the proposed theory, an attempt is possible. It will present new channels of investigation—for only by such means has progress been made in the past. As with the physical properties, the theory assumes no new mechanism of hardening.
Of the “resistance to flow” properties, hardness and yield strength are not easily correlated quantitatively. On the other hand, yield strength and tensile strength may be compared, since they are both properties of the stress-strain curve.
Ductility
The pronounced decrease in ductility does not originate wholly in the coherent state. Aging at room temperature promotes little or no change. At somewhat higher aging temperatures a slight decrease occurs. This is considered to be the change associated with coherency hardening. On the other hand, the pronounced decrease which occurs during aging at elevated temperature before maximum hardness is attained must be caused by overaging at grain boundaries.
Resistance to Fracture
The resistance to fracture or breaking stress is even more dependent than tensile strength on ductility and strain hardening in the tensile test. The few existing data indicate that the coherent state is characterized by an increased resistance to fracture. Fracture strength increases during the room temperature aging of duralumin when ductility is little changed.
Much emphasis has been placed on the significance of the overaged and weakened layer of solid solution adjacent to grain boundaries of alloys aged at elevated temperatures.
An Evaluation
In the proposed theory the only new quantities arc the characteristics of the coherent state. These combined with well- known reactions—solid solution hardening, recrystallization, dispersion hardening and coalescence, some of which can be isolated—are used to define the aging curves. The logical objection will be that the theory is so flexible that by arbitrary manipulation of the curves for the contributing reactions any aging curve can be reproduced.
This is not a justifiable objection judging from our knowledge of structure—limited as it is compared with the great mass of data which has accumulated on the changes in mechanical properties of the many precipitation alloys. To make a series of hardness measurements and thus establish an aging curve is a simple matter. But to determine the mechanism of precipitation for an alloy system or the rates of diffusion, nucleation and growth—all fundamental to the reaction—involves considerable effort.