Manganese Products, Inc. has developed a sulphurous-sulphuric acid leaching process for the recovery of manganese from low-grade, intermediate manganese ores. By the term “intermediate manganese ores” is meant those ores in which the manganese in the form of manganese oxides is found to have two different valences, usually two and four, as is probable in Mn3O4, or the ore may be a mixture of manganous and manganic oxides. “Intermediate” manganese siliceous ores are ores in which the silica is a constituent of the ore and is loosely linked to the manganese by water of crystallization.
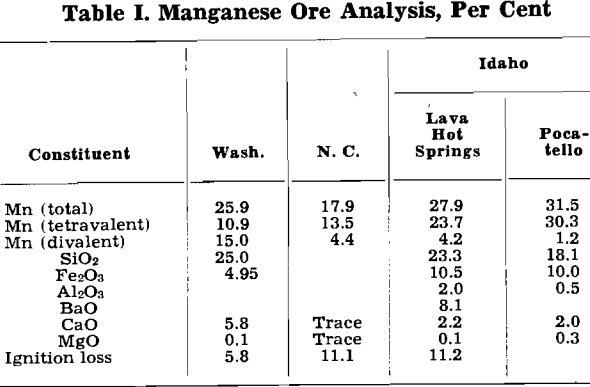
As far as the manganese leaching process is concerned, these ores differ primarily in the form in which the manganese oxides occur. The tetravalent manganese, the manganese existing in the dioxide form, is soluble in a sulphurous acid solution. To dissolve the divalent manganese, sulphuric acid is required.
To dissolve the type of ore found in Washington and North Carolina, a mixture of both sulphurous and sulphuric acid is used. Whereas to dissolve the type of manganese ore found in Idaho and also in Montana, sulphurous acid alone is sufficient. The Nevada manganese ore is of the same type as that found in Idaho as far as the leaching process is concerned.
Leaching of Ore: The manganese ore, ground to —65 mesh is leached with sulphurous acid and sul-
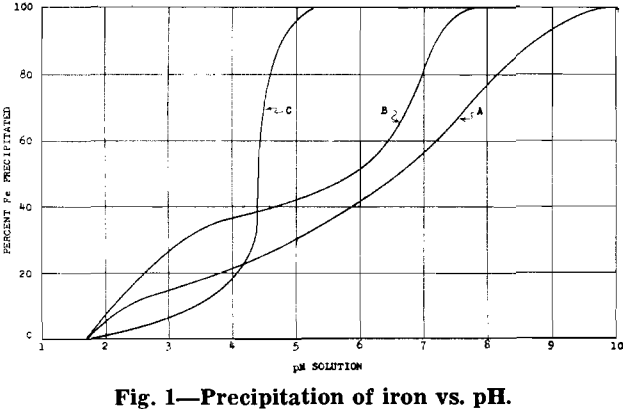
phuric acid at a temperature of 60°C. The sulphurous acid dissolves the quadrivalent manganese in the ore as is shown by the following equation:
MnO2 + H2SO3 → MnSO4 + H2O
2H2SO3 + O2 → 2H2SO4
MnO + H2SO4 → MnSO4 + H2O
MnO2 + 2SO2 → MnS2O6
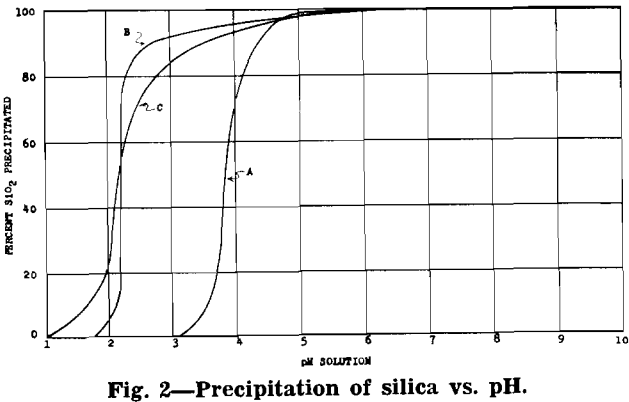
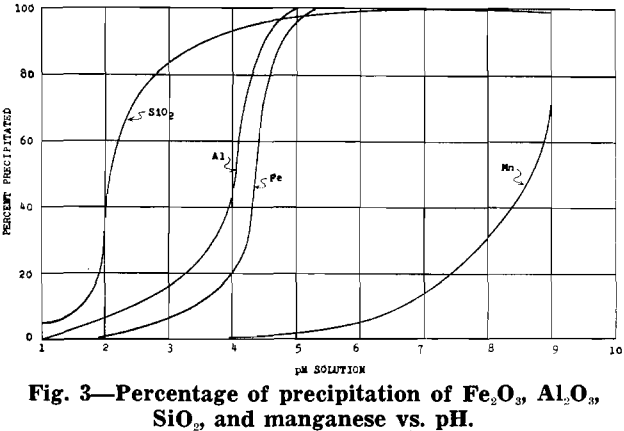
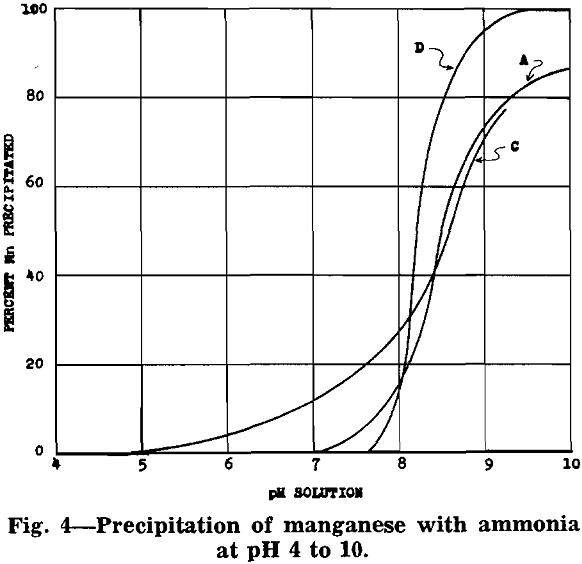
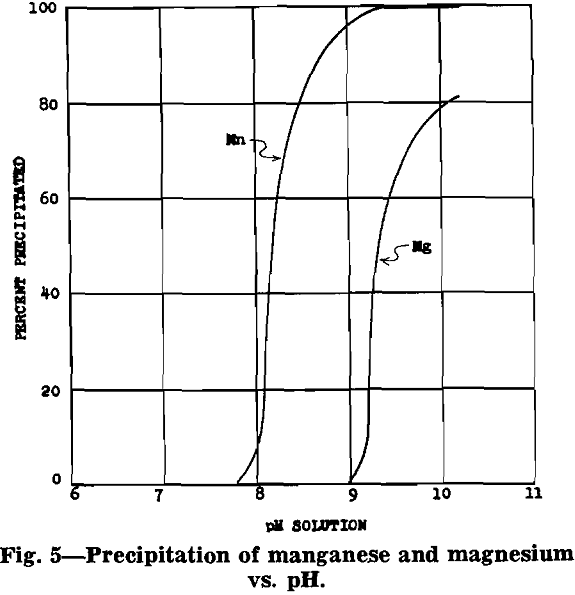
After removal of impurities, the solution is essentially manganese sulphate with some ammonium sulphate (formed from ammonia precipitation of impurities). Under proper control a manganese oxide product, in which 70 to 80 pct of the manganese is in the dioxide form, will precipitate from the manganese sulphate solution.
MnSO4 + 2NH4OH → MnO + (NH4)2SO4 + H2O
2MnSO4 + O2 + 4NH4OH → 2MnO2 + 2(NH4)2SO4 + 2H2O
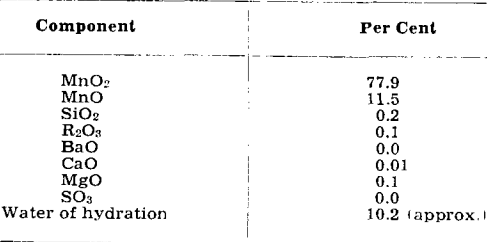
The equations show that an excess of ammonia must be used to insure complete precipitation of the manganese. The following analysis is typical for the product of this process:
Ammonium Sulphate Production:

The present plan, however, is to recover the ammonium sulphate. When the manganese ore is leached to a concentration in solution of 100 g Mn per liter, the equivalent ammonium sulphate formed is 24.7 lb per 100 lb of water. This is neglecting the ammonium sulphate formed from the precipitation of the impurities of iron, alumina, and silica. Ammonium sulphate can be recovered several ways: (1) addition of outside heat to evaporate the water after the excess ammonia has been stripped from the solution, (2) neutralization of the excess ammonia with sulphuric acid and addition of outside heat to evaporate the water, or (3) addition of enough sulphuric acid and ammonia and taking advantage of their heat of reaction to affect crystallization of ammonium sulphate.
Taking a basis of 100 tons of 30 pct Mn ore per day and assuming 90 pct recovery of Mn, 451.8 tons is the theoretical ammonium sulphate production if all the water is evaporated.
Primarily because of the shortage of sulphuric acid at the beginning of operations, the excess ammonia will be stripped from the solution, and the water will be evaporated to crystallize ammonium sulphate.
