Table of Contents
The recent increase in anode copper production at the Noranda smelter placed an additional load on the Montreal East plant of Canadian Copper Refiners Limited. The consequent increase in the amount of anode slimes led to a careful consideration of the various methods of slimes treatment.
Characteristics of the Slimes
Anode slimes are made up of those components of the anodes which are not soluble in the electrolyte. They contain varying quantities of copper, silver, gold, sulphur, selenium, tellurium, lead, arsenic, antimony, nickel, iron, silica, etc.
Copper in the slimes originates to a large extent in the cuprous oxide of the anodes and is therefore dependent on the oxygen content of the latter:
Cu2O + H2SO4 → CuSO4 + H2O + Cu………………………………………..[1]
Cu2SO4 → CuSO4 + Cu……………………………………………………[2]
The product of either reaction is very finely divided.
Depending on the slimes composition, much of the copper is combined with sulphur, selenium, tellurium, etc. The balance of the copper is in the free metallic state.
Treatment of Slimes Components
Direct smelting of slimes is no longer practiced. Although a high initial gold fall is obtained, excessive matte and slag formation causes heavy recirculation of precious metals, especially of silver. A much more satisfactory approach is separating the component elements one by one. In this manner they may be recovered in a reasonably pure state permitting either immediate withdrawal from the circuit or recirculation with a reduced number of treatment steps.
Removal of Copper: As a rule, copper is removed as a water soluble sulphate. In this form it may be sent directly into the electrolyte circulation system. However, due to the presence of impurities, such as selenium and tellurium, this is practiced in few instances only. Generally, the leach solution is worked for the recovery of copper sulphate, or, if the impurities content is too high, sent to the liberator system.
In few cases only are the liberator cathodes of commercial quality. They are usually remelted in the anode furnace. The liberator solution may be concentrated although the strong acid is quite impure and usually not suitable for electrolytic work.
Following are the commercial methods for recovering copper:
Oxidizing Roast: Shallow layers of slimes are heated to 500-800°F. Sufficient air is admitted both to effect oxidation and to hold the temperature below the fritting point. The bed is generally not rabbled to avoid dust losses although if efficient dust collection is present, rabbling is employed to reduce the roasting time. Copper compounds are broken down and together with free copper form copper oxide, selenite and tellurite. Some selenium and arsenic are volatilized. The calcine is leached with dilute sulphuric acid, foul electrolyte or liberator solution. The solution is then sent to electrolytic department, copper sulphate recovery or liberator system, as discussed above.
Sulphatizing Roast: This method is an improvement over air roasting in that concentrated sulphuric acid is added to the slimes prior to charging or during the roast by spraying. The acid acts both as an oxidizing and a sulphatizing agent.
Cu + 2H2SO4 → CuSO4 + 2H2O + SO2……………………………………[3]
Other metallic components react similarly. Selenium and tellurium are oxidized to the corresponding dioxides or oxysulphates
Se + 2H2SO4 → SeO2 + 2H2O + 2SO2………………………………………..[4]
Te + 2H2SO4 → TeO.SO3 + 2H2O + SO2…………………………………….[5]
Since the acid is required both for oxidation and for sulphation of the copper, its requirement is double that used in methods described under Oxidizing Roast and Aeration in Dilute Sulphuric Acid.
Roasting at high temperature drives off the selenium dioxide. To prevent decomposition of copper sulphate, temperature must not exceed 1200 °F. Yet at C.C.R., roaster temperatures have reached 1400 °F, with no noticeable effect on copper sulphate.
The roasting step may be modified by a preliminary heating of the slimes-acid mixture below the boiling point of the acid. The sulphur dioxide and water given off and the base metal sulphates formed increase the porosity of the mix and aid in retaining the acid needed to complete the oxidation of selenium and tellurium at a higher temperature. Roasting then merely drives off the volatile oxides.
The roasted slimes are leached with hot water. The leach liquor carries much silver and some selenium and tellurium. Silver is readily cemented on metallic copper or raw slimes. Selenium requires a somewhat longer cementation time, while tellurium is more resistant to cementation than selenium. Both require an acid environment.
The reactions involved are complex, but may be represented by the overall equation
4Cu + 2H2SO4 + H2SeO3 → 2CuSO4 + Cu2Se + 3 H2O………………………………….[6]

Removal of Selenium: Wherever selenium occurs in appreciable quantities, market value makes its recovery attractive. In consequence, the slimes treatment flow sheet must be chosen with a view of greatest and most economic recovery of the element in the purest state possible. Actually, many compromises must be made.
Selenium recovery is about 80 pct due to inevitable losses in mattes, slags and flue dust.
Removal of Tellurium: Tellurium is eliminated from the slimes as a water soluble sodium tellurite. It is converted into this form after a preliminary oxidizing or sulphatizing treatment. That may be done in several ways: (1) roasting or baking a slimes-soda mix—this is a combined oxidizing and “alkalizing” treatment; (2) refining with soda in Dore or cupelling furnace; (3) boiling the slimes with caustic soda after an oxidizing or a sulphating roast.
Sodium tellurite is extracted by water leaching the roasted product of (1) or the soda slag from (2). In all three cases the liquor also contains sodium selenite. Boiling the sulphate roasted slimes extracts some lead in addition to selenium and tellurium.
Whatever the method, the aqueous solution is neutralized to pH 6-6.2 to precipitate flocculent tellurium dioxide, or tellurium mud. This is purified by redissolving in caustic soda, precipitating the impurities with sodium sulphide or sodium sulphite and neutralizing again to obtain tellurium dioxide of higher purity. In a method formerly used at C.C.R., the mud containing copper and selenium as impurities was roasted with sulphuric acid to volatilize the selenium and then water leached to remove the copper.
There are three methods for recovering elemental tellurium from the mud: (1) direct reduction by heating with flour under a borax cover; excessive fuming of tellurium dioxide is a disadvantage; (2) dissolving the mud in hydrochloric acid or in sulphuric acid to which some salt is added, followed by reduction with sulphur dioxide, filtration, washing, drying and melting (3) dissolving the mud in caustic soda and electrolyzing with iron anodes and stainless steel cathodes.
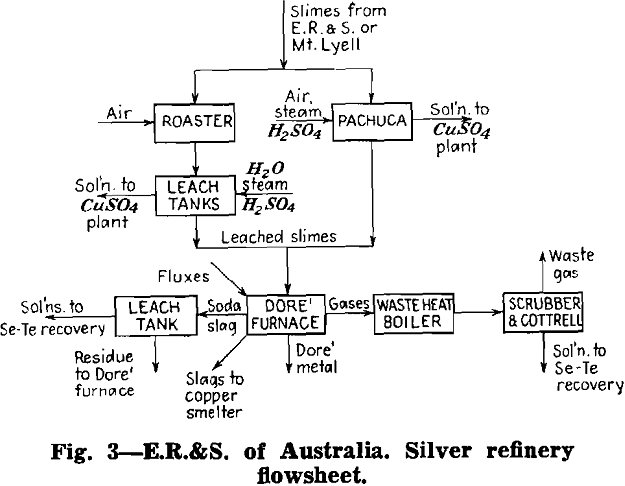
Silver Refinery Practice
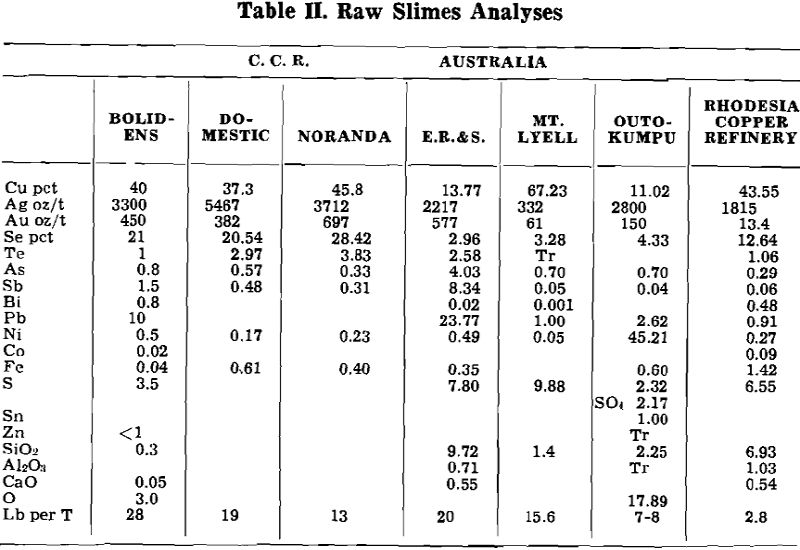
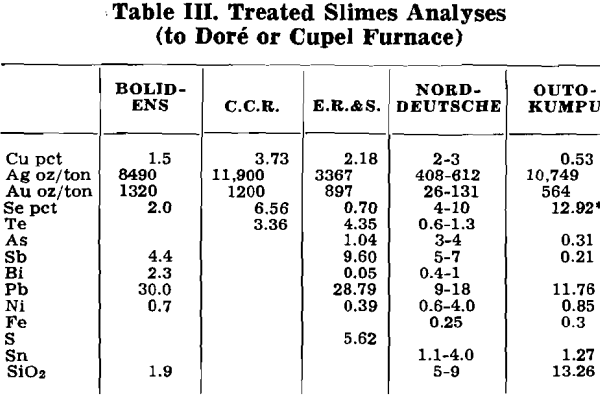
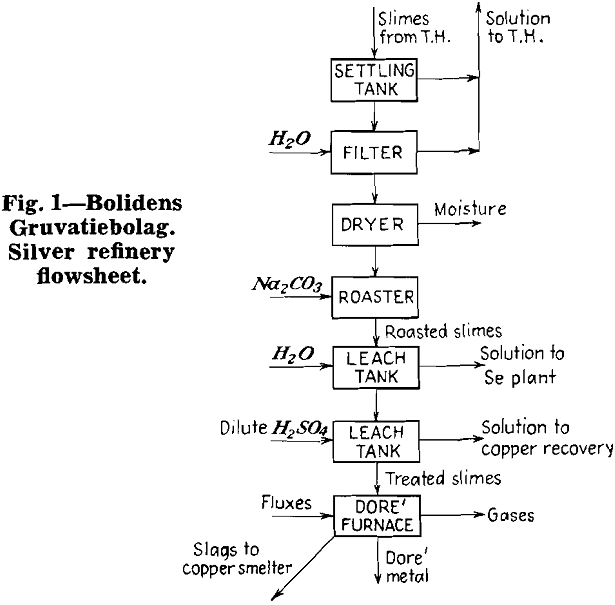
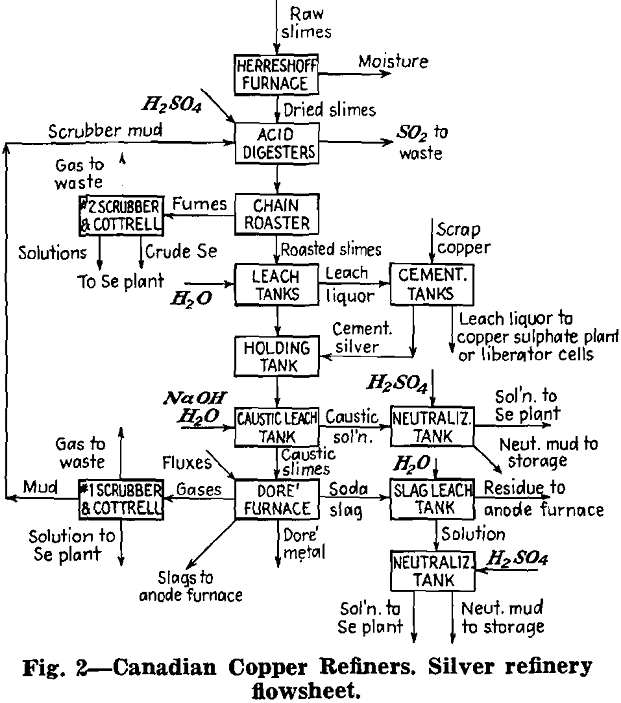
Herewith are presented the data supplied by co-operating refineries. Analyses of anode copper, raw slimes and treated slimes are given in tables I, II and III, respectively. Flowsheets are shown for each refinery.
Bolidens Gruvatiebolag, Ronnskar, Sweden: Boliden Mining Co. produces anode slimes of high selenium-low tellurium content, (21 pct Se, 1 pct Te). Because of this combination, the first step in the flowsheet is a soda ash roast which is followed by a water leach to eliminate the selenium (fig. 1).
0.45 ton Na2CO3 is mixed per ton dried raw slimes. The mixture is roasted in a small multiple hearth furnace, at a maximum temperature of 840 °F. Since temperature control is important, the furnace is electrically heated by resistors fixed to the hearths.
The soda-baked calcine is water leached in propeller-agitated tanks to remove selenium. The leach solution as press-filtered contains about 52 gpl Se and 32 gpl Na2CO3. This solution is treated for selenium recovery.
Fused soda ash is then fed to the furnace, and the charge is blown with compressed air. The soda slag formed contains most of the selenium and tellurium from the charge. This slag is leached with water to dissolve the selenium and tellurium content. The residue from the slag leach is returned to the anode furnace. The alkaline slag leach filtrate is neutralized with sulphuric acid which precipitates tellurium, while the selenium remains in solution and is recovered at the selenium plant by SO2 precipitation. The neutralized mud is also being stored at the present time.
The final stage of Dore furnace refining is the removal of the residual copper content by rabbling the charge with niter. The copper slag is skimmed off, crushed and returned to the anode furnace.
Four materials are received at the selenium plant for processing: crude selenium from the chain roaster scrubber, neutralized slag leach solution, and two types of scrubber solution. Typical assays of the materials are given below:

Parting Plant Practice: As noted in the introduction, the second portion of the paper describes methods of treatment of raw slimes up to the production of Dore metal. To complete the data, this final section covers electrolytic parting plant practice in general, although figures for two actual plants are used as illustrations.
Anodes are removed from the Moebius cell bags after 24 to 28 hr, scraped to remove the passive gold film and replaced in cells for an additional 4 to 6 hr. When necessary, scrappy anodes are replaced in cells by new ones. Once every four days all anodes are removed, the bags are drained and emptied and the scraped anode scrap is remelted in a graphite crucible and cast into Dore anodes for parting.
The gold mud scraped from the anodes is combined with that from the bags, washed with water and boiled with concentrated sulphuric acid to remove silver and selenium. The acid leached gold is washed with water and drained on Filtros blocks. The gold sand is melted in a crucible furnace and cast into bars assaying 998+ fine Au.

