Table of Contents
Pyrrhotite has long been considered a gangue mineral to be eliminated as tailing in the treatment of various sulphide ores. However, in recent years the world-wide lack of sulphur resources has called attention to this mineral as a potential source of both sulphur and iron. Its importance as an economic mineral, however, has not been particularly emphasized.
Preparation of the Pyrrhotite Sample: It was desirable that the highest grade of pyrrhotite obtainable be used for this experiment, since the presence of other minerals could affect the surface properties. However, no pyrrhotite was available as crystals, and massive deposits of hydrothermal origin commonly contain considerable amounts of chalcopyrite. Pyrrhotite concentrate was, therefore, prepared from a sulphide deposit occurring near Aitkin, Minn. The deposit is of pyrometamorphic nature consisting mainly of pyrrhotite and pyrite with graphite, silicates, and carbonates as gangue.
After each flotation test, the cell was thoroughly washed with hot water and rinsed with demineralized water. Occasionally the cell was boiled with aqua-regia to remove fine mineral particles plugging the pores of the fritted glass.

Experimental Results
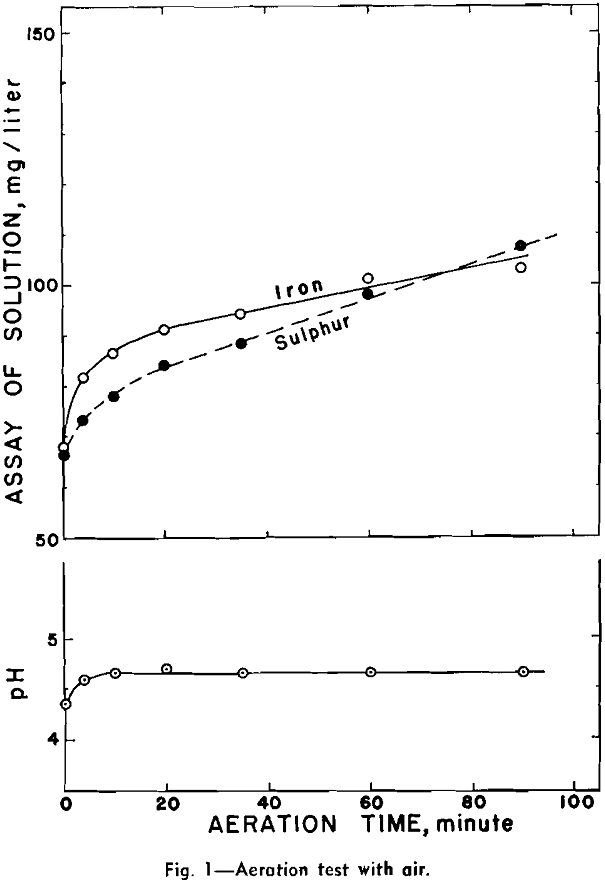
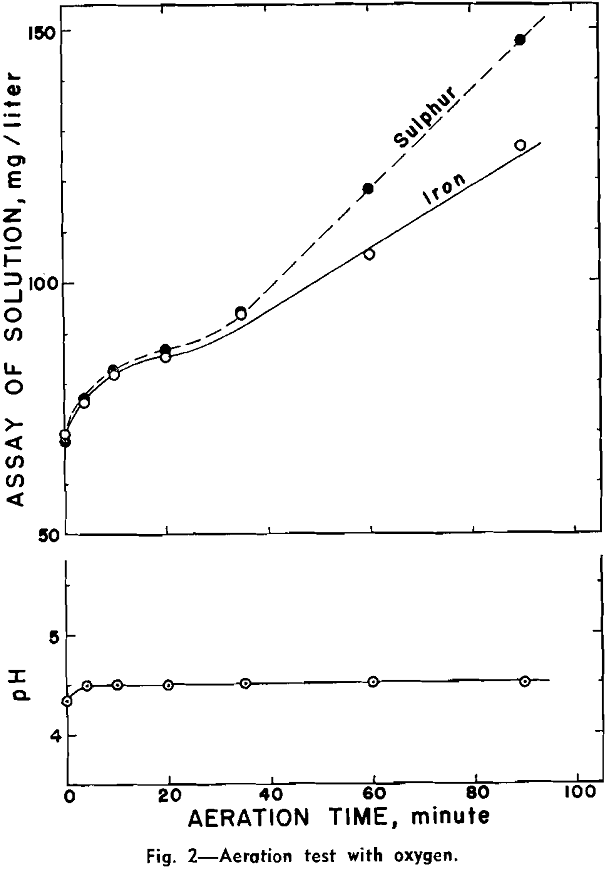
Aeration and Copper-Abstraction: A preliminary test was made for the presence of ferrous, ferric, and sulphate ions in the filtrate from an aerated aqueous suspension of pyrrhotite. .
Aeration at Natural pH: Three aeration tests were made with air, oxygen, and nitrogen respectively. These three tests were performed within one week to minimize any serious sample alteration during storage.
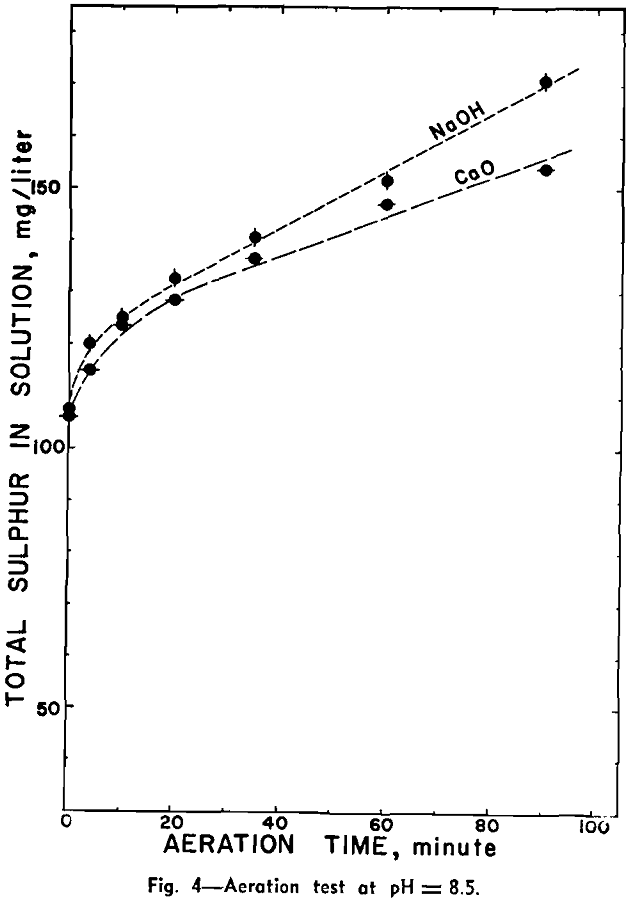
Aeration at Alkaline pH: A similar experiment was carried out with air in an alkaline circuit. The pH was maintained at approximately 8.5 by the addition of calcium hydroxide or sodium hydroxide at regular intervals.
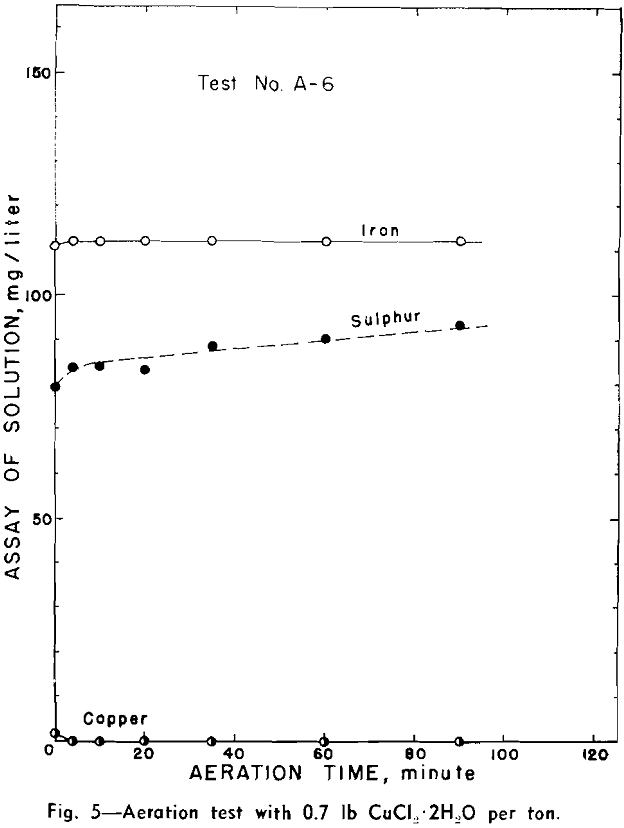
Aeration in Presence of Cupric Ion: Aeration tests with addition of 0.7, 3.4, and 6.9 lb of cupric chloride (CuCl2·2H2O) per ton were made. These represented initial concentrations of 14.4, 71.7, and 143.5 mg respectively of copper per liter of solution. Only air was used in this experiment.
Effect of Collector Addition With and Without Copper Activation: Two series of flotation tests were performed. Potassium ethyl xanthate was used as collector in one series and potassium amyl xanthate in the other. The collector addition ranged from 0.4 to 6.4 lb per ton of pyrrhotite. In two similar series of tests pyrrhotite was first conditioned with 1 lb cupric chloride per ton for 15 min prior to flotation.
Effect of Collector Addition in Stages: In this experiment, the collector was added in stages and float products were collected separately after each addition. The cumulative amount of collector at each stage was arranged to correspond with those of single addition in the previous experiment.
To study the size distribution, screen analyses were made on all flotation products obtained in these tests. The cumulative percent weight recovered in each size fraction is plotted in Figs. 11 and 12 against the cumulative amount of collector added.
The results show that the floatability of pyrrhotite decreases with increase in particle size. This tendency is most pronounced when ethyl xanthate is used as collector to float unactivated pyrrhotite with the +200 mesh particles responding very reluctantly to flotation. The results also confirm the increase in floatability with copper activation.
Detrimental Effect of Excess Cu++ ion in Pulp: Even though copper activation of pyrrhotite increases its floatability, the presence of excess Cu- ion in the pulp precipitates xanthate according to the following reaction, and the presence of excess Cu++ ion is undesirable.
4 ROCSS- + 2 Cu++ → 2 ROCSS · Cu + (ROCSS-SSCOR)
This is demonstrated by two flotation tests in which the pyrrhotite was conditioned with 5 lb of cupric chloride per ton for 15 min and 2 hr respectively and the amyl xanthate was added in increments.
In a separate test the equivalent of 6.4 lb of amyl xanthate per ton was mixed in the cell with a stoichiometric excess of Cu++ ion (equivalent to 3.5 lb of cupric chloride per ton) prior to the addition of pyrrhotite. Flotation with this mixture as a collector gave a recovery of approximately 18 pct, presumably due to the independent collecting ability of cuprous xanthate and dixanthogen, and may account for that flotation which takes place prior to the first inflection point.
The collector and the frother were added after 3 min conditioning with acid or with alkali. The pH of the pulp was measured before and after each flotation test. The average of the two values was taken as the pH during flotation. In the first series of tests total iron and sulphur analyses were made on the pulp solution prior to flotation.

for the increase in recovery with pH in that range. After the addition of acid or alkali, however, it was also noted that the pH tended to drift toward the equilibrium value. For this reason a series of different recoveries was obtained at the critical pH.
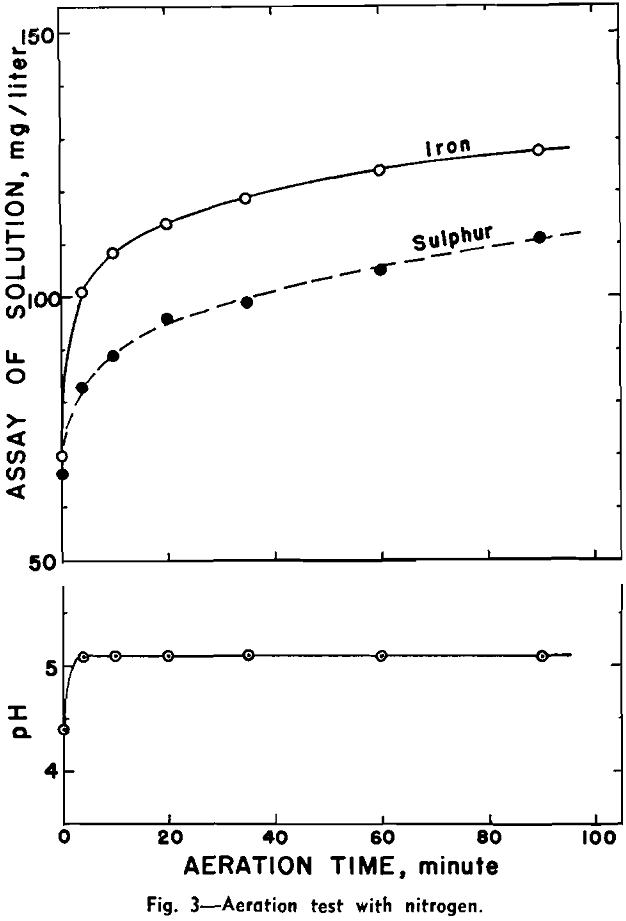
Effect of Aeration on Flotation: Flotation tests were made by first aerating different pulps with air, oxygen, and nitrogen each for 1 hr. Amyl xanthate, 1.6 lb per ton, was used as collector.
The results show that aeration with air and oxygen reduces the floatability of pyrrhotite. There is no great difference in the amount of iron and sulphur present in the three pulps.
Discussion of Results
Cupric ion is abstracted by pyrrhotite in the presence of air and its depletion from solution is proportional to the increase of iron in the pulp. The resulting activated pyrrhotite ceases to liberate iron and sulphur on further aeration. However, a considerable amount of copper is abstracted from solution in the first 15 sec of agitation.
The results show, within experimental error, the stoichiometric exchange reaction between copper ion and iron atoms at the pyrrhotite surface.

