High temperature-high pressure techniques have long been used to great advantage in the organic chemical industry, the petroleum industry, and the paper industry. Only recently, however, have these methods been used to extract metals from their ores on a commercial scale. The Chemical Construction Corp., together with interested producers of nickel and cobalt, has done much to develop methods of producing nickel and cobalt powders by the use of high temperature-high pressure techniques.
Equipment: The high temperature-high pressure reaction unit used in this work was especially designed for kinetic studies of this nature, emphasis being placed on accuracy of measurement and precision of control. As details of the unit’s construction and operation have been discussed elsewhere, only basic features and principles of operation will be discussed at this point.
- Temperature of the solution within the autoclave is constantly measured, recorded, and controlled.
- Gas over-pressure of the system is constantly regulated and indicated.
- Provisions are made for lowering a sample into the reaction zone of the autoclave once thermal equilibrium has been attained.
- A means is provided for removal of liquid samples from the reaction zone of the autoclave during the course of the reaction without disrupting operation of the unit.
Experimental Procedure: Molybdenum sulfide used in the kinetic study was Molysulfide lubricant, Grade 2, a commercial product manufactured by Climax Molybdenum Co. The MoS2 content of this material was better than 99 pct. A less pure grade of molybdenite, obtained from the Kennecott Copper Co., was used for the bench-scale tests. This material contained approximately 89 pct MoS2, the remainder being a siliceous gangue material.
Molybdenum content of the leach solution, measured at periodic intervals, was determined spectrophotometrically from a phenylhydrazine hydrochloride-molybdenum complex by a Beckman Model DU quartz spectrophotometer. The method of analysis followed that developed by Ayres and Tuffly.
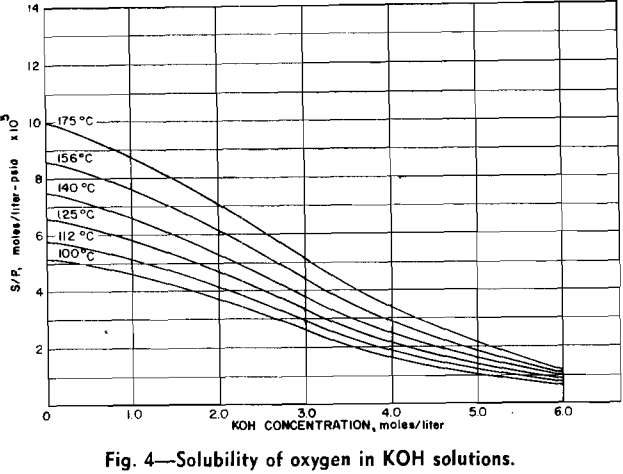
Measurements of oxygen solubility in the leach solutions were made with the equipment previously described. Since a relatively large volume of solution was required for each solubility measurement, it was not possible to measure solubility concurrently with molybdenum concentration. When a solubility measurement was made, the solution within the autoclave was brought to the desired temperature and the oxygen over-pressure was increased in increments of 100 to 200 psi. A
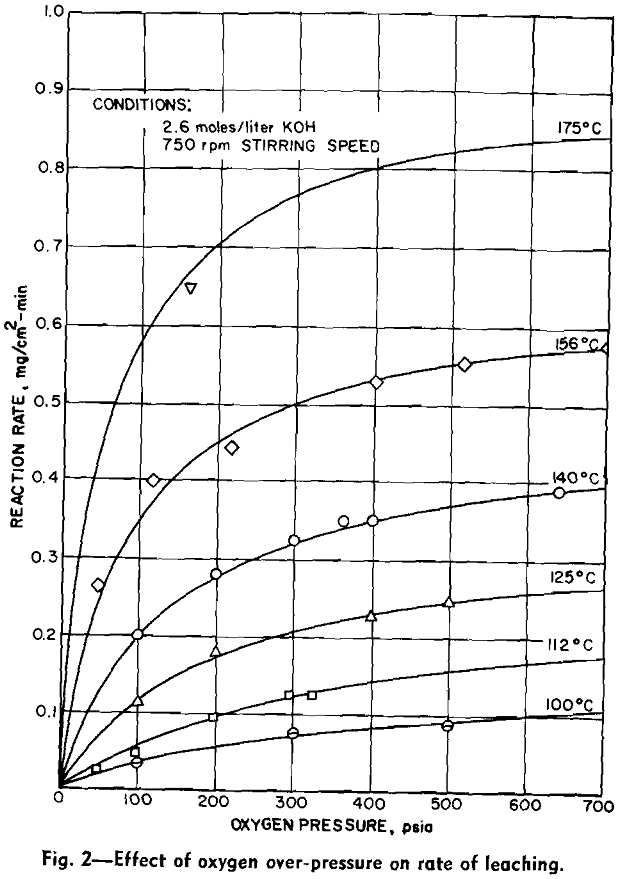
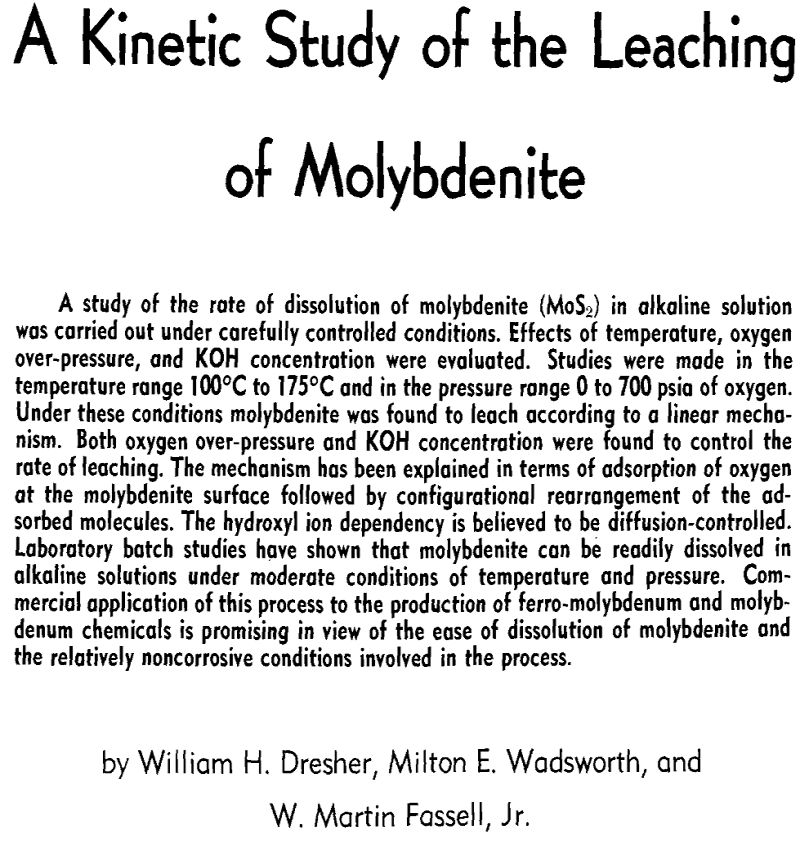
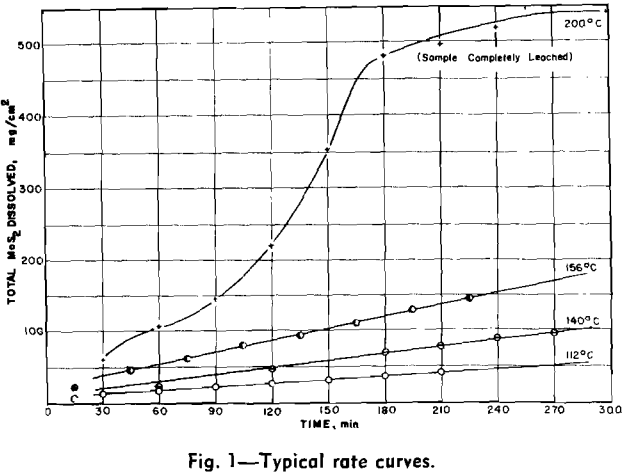
Experimental Results: The reaction rate data consisted of measurements of the rate of formation of MoO4– under the various conditions of leaching. Results to be described are based on the leaching of more than 65 samples of molybdenite in potassium hydroxide solution under oxygen partial pressure of 0 to 700 psia and at temperatures from 100°C to 175°C.
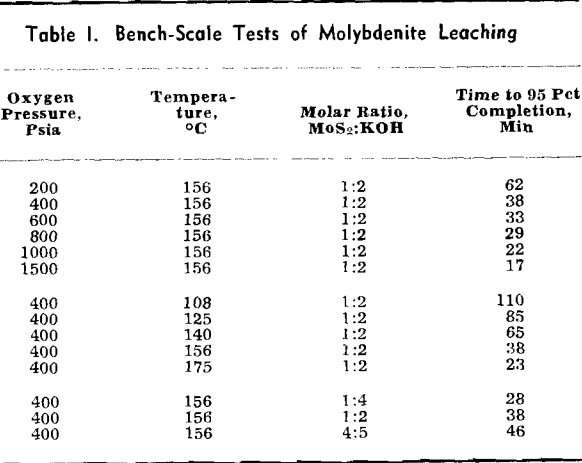
Four variables were studied in determining kinetics of the leaching reaction: speed of agitation, KOH concentration, temperature, and oxygen overpressure. Rate of the reaction was grossly affected by speed of agitation below approximately 500 rpm. Above this speed, however, reaction rate was relatively stable until cavitation occurred at about 1000 rpm and the sample disk was no longer covered by the leach solution. Stirring rate was maintained at 750 rpm for all tests.
The overall measured rate is clearly a function of the oxygen over-pressure. The shape of this curve suggests a surface adsorption process that reaches a maximum rate for complete surface coverage. The oxygen dependency curves are regular and typical of a process involving adsorption of a gas onto a surface. Hydroxyl ion dependency, however, is not so simple. The isotherms can be divided into two regions. In the first region, below a concentration of approximately 1.0 mols per liter, the reaction rate is an increasing function of the hydroxyl ion concentration. Above this region the rate decreases with increasing hydroxyl ion concentration.
The overall reaction may be explained stoichiometrically by
MoS2 + 9/2O2 + 6OH- → MoO4– + 2SO4– + 3H2O………………………………(1)
After complete oxidation the end products were the molybdate and the sulfate ions. If, however, the solutions were analyzed as soon as they were removed from the autoclave and the molybdenum sulfide was not completely reacted, the sulfur was found to be present in solution as the sulfate ion plus a measurable quantity of the thiosulfate ion —S2O3–
MoS2 + 5/2O2 + 4OH- → MoO4– + S2O3– + 2H2O………………………………(2)
The effect of varying hydroxyl ion concentration is irregular from what might be expected. The concentration of oxygen dissolved in the KOH solution was not a constant due to the variation of the solubility of oxygen in the various concentrations of KOH. The effect of varying the hydroxyl ion- concentration can be explained by considering the two sections of each of the isotherms separately. In the first section the solubility of oxygen in the dilute KOH solution is sufficient to supply an adequate amount of oxygen to the surface of the molybdenite in order to support the reaction. In this section, however, the hydroxyl ion does not diffuse to the surface fast enough to continue the reaction. Thus the rate of the reaction increases as the hydroxyl ion is increased.
The product of the leaching process is a pregnant liquor carrying the desired molybdenum plus any other alkali-soluble metals that may have been present in the ore. Such a process, if applied to molybdenite ores containing appreciable quantities of rhenium, would result in a liquor containing potassium perrhenate as well as potassium molybdate. This liquor could then be treated by ion exchange or by solvent extraction to separate the rhenium from the molybdenum.
The sands flotation treatment in the molybdenum circuit, such as is currently used to separate molybdenum sulfide from the underflow of copper flotation circuits, could be eliminated by application of this process for dissolution of molybdenum. The depressed molybdenite plus the gangue material removed following the bulk sulfide step should be directly applicable to such a leaching process.