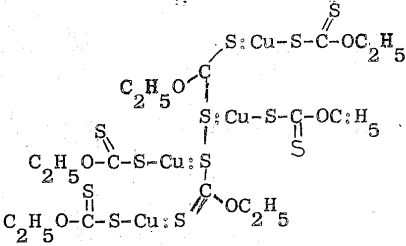The flotation system is usually distinguished from other physical chemical systems by the importance of interactions at the solid-liquid and solid-gas interfaces. In recent years, numerous quantitative studies have been made of the distribution of common collectors between the solid and aqueous solution phase. Although in flotation the main emphasis is placed on the adsorption process, chemical reactions will also take place in the bulk solution phase. These side reactions are often undesirable and will contribute towards the complex behavior of many a flotation system.
Experimental Methods
Two electrochemical methods, a conductometric and a potentiometric titration technique were used to determine the solubility products of zinc, lead, silver, copper and ethylxanthate salts.
(a) Conductometric Method: This method is based on the determination of the specific and equivalent conductance of a saturated solution of the metal xanthate under investigation. The solubility of the salt may be calculated from the relationships

where K expressed in ohm-¹ cm-¹ is that part of the specific conductance of the solution due to the ions supplied by dissociation of the salt which has a concentration c equivalents per liter and Λc is the equivalent conductance in ohm-¹ cm² of the salt. Equation (1) after a slight rearrangement of terms is used to define the equivalent conductance of an electrolyte.
![]()
where lo- and lo+ represent respectively the limiting equivalent conductances of the anionic and cationic constituents of the particular salt. With this approximation and from the experimentally determined value of K, the saturation concentration may be calculated. This calculated value of c is then used to determine Λc by substitution in the Onsager Equation which for bi-univalent electrolytes in water at 25° C assumes the form,
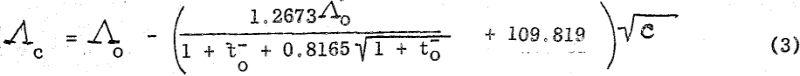
where to- (=lo-/Λo) is the limiting transference number of the anion. By substituting now the calculated Λc value in Equation (1) a more accurate value of c may be determined. This process of successive approximation is then repeated until a satisfactory value for c is obtained.
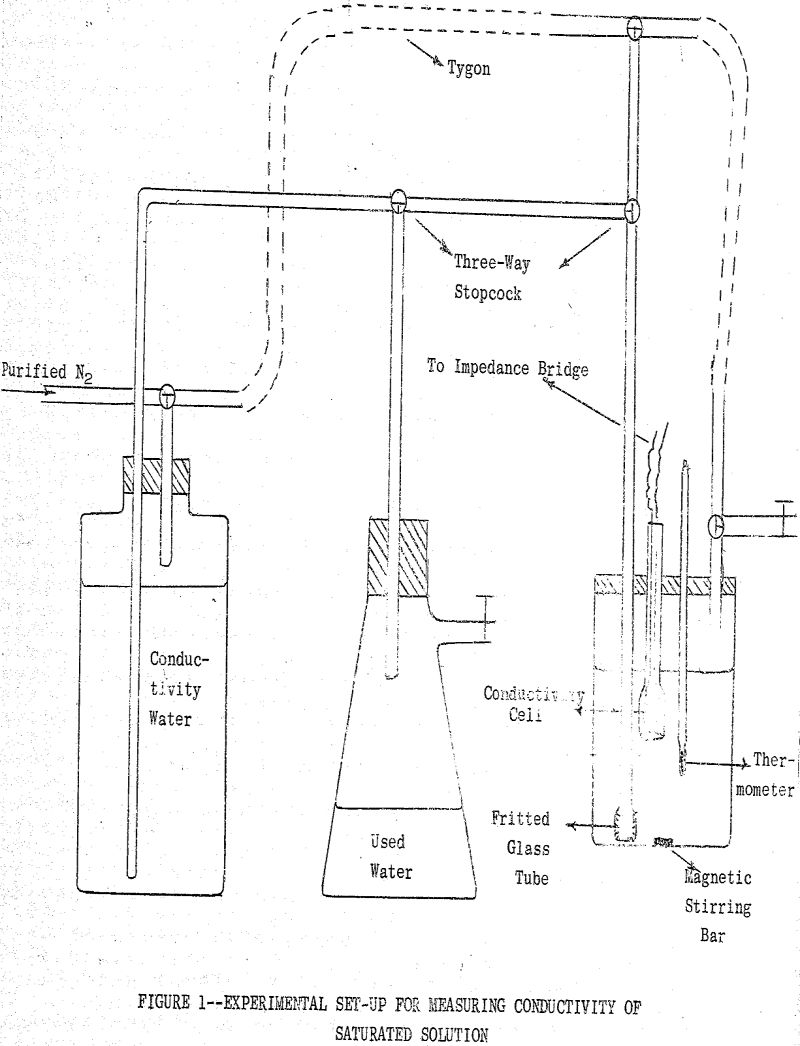
Samples of solid metal ethylxanthates for conductivity measurements were prepared by the following procedure: To 400 ml, of a solution of 3.5 g. of freshly purified KEX conductivity water (~0.05 N) was added 200 ml. of a metal nitrate solution of approximately the same normality while stirring the solution. The precipitate was filtered and washed thoroughly with conductivity water and was then stored under conductivity water in a closed flask.
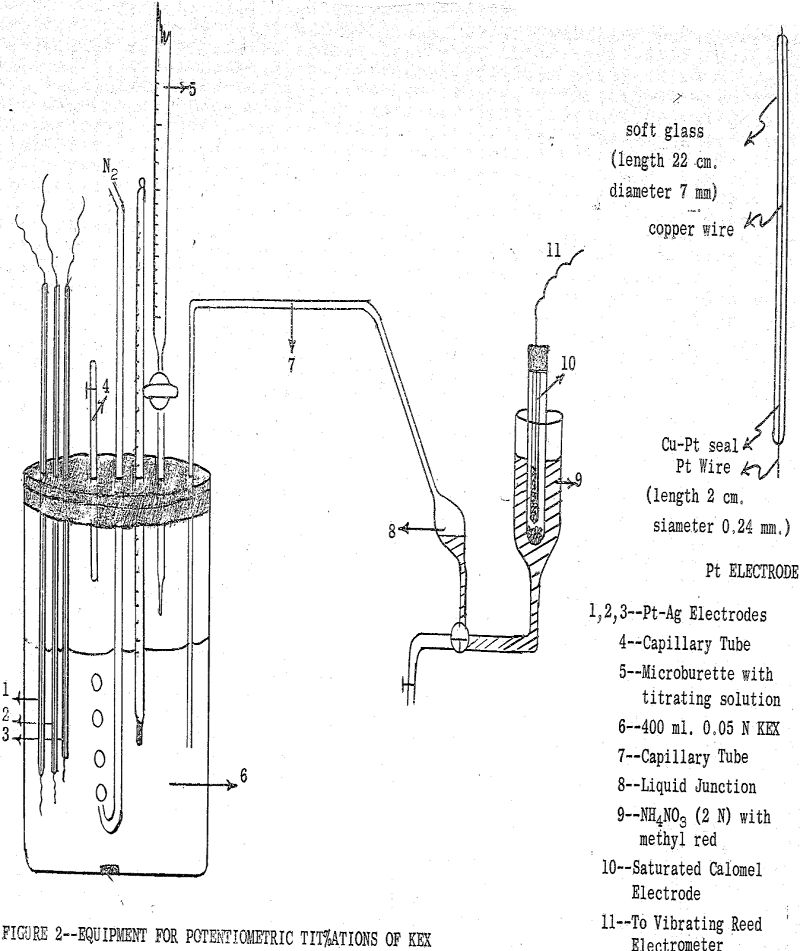
(b) Potentiometric Method: This method involves a potentiometric titration of an aqueous KEX solution with a soluble salt of the metal of interest. The reversible electrical cell used in this titration contained a reversible silver-silver ethylxanthate electrode and a saturated calomel electrode as reference electrode.
To determine the solubility products of other metal ethylxanthates, the aqueous KEX solution was titrated first with AgNO3 and then the titration was continued with a solution of the salt of the metal of interest.
Experimental Results
(a) Limiting Equivalent Conductance of EX-:The limiting equivalent conductance of EX- at 25° C as determined from these plots by extrapolation and interpolation is 41.5 ohms-¹ cm². In arriving at this value, a limiting conductance of 74.5 ohm-¹ cm² for K+ at 25° C was taken. In making these calculations, the effect of hydrolysis on the equivalent conductance was neglected because even at 10 -4 KEX the degree of hydrolysis is only about 10 -4.
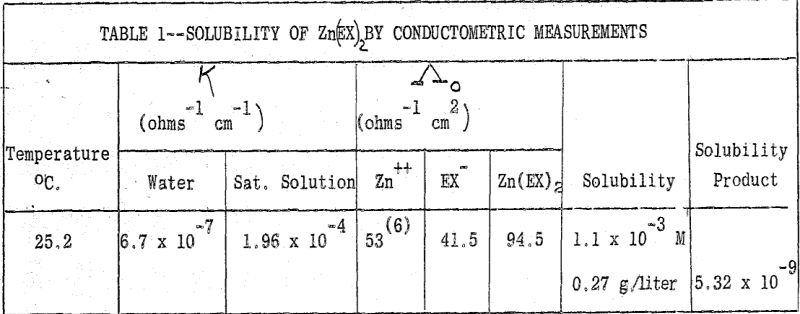
(b) Solubility of Zinc Ethyl Xanthate: The solubility of this salt was determined by the conductometric method. The solution reached saturation in 25 minutes after addition of Zn(EX)2. Three independent measurements gave the same value for the specific conductance of the saturated solution.
Solubility of Silver Ethylxanthate: In the determination of the solubility product of AgEX by the potentiometric method, 400 ml. of a 5 x 10-³ M KEX solution was titrated with a 0.1 N AgNO3 solution. Two separate titrations were done. One of the titration curves obtained is shown in Figure 4 where the EMF of the cell measured relative to the saturated calomel electrode is plotted against ml. of AgNO3 solution added.
Decomposition of Cupric Ethylxanthate
Cupric ethylxanthate is known to decompose readily to cuprous ethyl-xanthate. This decomposition may be explained by the following oxidation-reduction reaction in the solution:
2 Cu²+ + 2 EX- ↔ 2 Cu+ + (EX)2
Three distinct possibilities exist regarding the composition of the precipitate formed during this titration. The precipitate may be (a) only Cu(EX)2 or (b) only CuEX or (c) a mixture of Cu(EX)2 and CuEX.
If the precipitate formed during the potentiometric titration is Cu(EX)2, then it may be concluded that its solubility product is 4.4×10 -27 as indicated in Table 5 and that the solubility product of the cuprous salt must be greater than 10 -11.72 x 10 -7.3 or 10 -19. On the other hand, if the precipitate is CuEX then its solubility product will be approximately 10 -19 and that of the cupric salt will be greater than 4.4 x 10 -27. If both copper xanthates are present in the precipitate, then it follows that the solubility products are 4.4 x 10 -27 and 10 -19 for the cupric and cuprous states respectively.
The results of the gravimetric analyses may also be explained by postulating the composition, 4CuEX.(EX)2, for the solid. This compound might be formed by complex formation between cuprous xanthate and ethyl-dixanthogen produced by the oxidation-reduction Equation (8). Its structural formula may be written as follows: