Table of Contents
Generalized conclusions cannot be drawn from the laboratory studies about the economic feasibility of extracting mercury by a combined flotation and fluidized-bed roasting process.
The 94 percent mercury recovery indicated in the laboratory compares favorably with the 90 to 95 percent recoveries usually reported by mercury-plant operators. Grinding the entire ore to liberation size, plus the cost and operation of a concentrator, is a disadvantage of any two-process treatment. Conversely, the relatively small roasting plant required, the fuel saved because a large amount of gangue is rejected before roasting, and the possibility of obtaining an antimony by-product from some ores must be listed as advantages of the combined method. Labor conditions, transportation costs, and nature of the individual ore all have a bearing when selecting a treatment method. It appears, however, that especially in areas of high fuel costs, the two-process method warrants careful consideration.
Conventional extraction of mercury from cinnabar involves only oxidizing roasting of the ore to burn the sulfur and to volatilize the mercury, subsequently recovered by cooling and condensation. The ore usually is roasted in rotary kilns or multiple-hearth furnaces. When the ore is fine or where the operation is small, however, simple externally heated retorts are used. The process is efficient, and under ordinary conditions the cost is nominal. Concentration of mercury ores before roasting has not been widely adopted, because the cost of the additional treatment and the losses in concentration usually offset the cost of furnacing the entire ore.
Under certain conditions, however, such as operation in areas of high fuel costs, concentration conceivably can be economic. In 1957 the Arentz-Comstock mine in Oregon began producing with a flotation-retort process. The design of the flotation plant was based upon tests at the Albany (Oreg.) laboratories of the Bureau of Mines. During the same year John Etchart began producing at a flotation plant in the Bottle Creek area of Nevada.
The possibility that recovery of an antimony byproduct would offset additional costs of treatment led to a preliminary investigation of concentration of cinnabar-stibnite ores. The results of this study indicated that a possible recovery process included bulk flotation of the sulfides, followed by oxidation roasting with close control of temperature and atmosphere. A similar process was employed by Menardi on livingstonite concentrate.
This report summarizes the results of substantiating tests to evaluate the applicability of concentration-roasting techniques for treating Alaskan mercury antimony ores. Included are descriptions and results of small-scale continuous flotation tests and of fluidized-bed roasting tests conducted on cinnabar-stibnite concentrate and on antimony-free cinnabar products. Only the mercury-recovery phase of the problem has been investigated. Results of studies to determine an economic method for recovering byproduct antimony will be reported in another paper.
This investigation was directed toward demonstrating the metallurgical feasibility of the combined concentration-roasting process. No attempt was made to establish optimum operating conditions or to determine the economic practicability of the treatment method.
Concentration
The Ore
Ore for this investigation was obtained from the Red Devil mine near Sleetmute, Alaska; careful selection was made to obtain material as nearly representative as possible of the known ore body. The Red Devil property is Alaska’s major producer of mercury. In 1957 it supplied approximately 16 percent of the total quicksilver output of the United States. The ore is purported to be typical of ores widely distributed throughout the entire Kuskokwim River Basin; in their aggregate they may constitute large potential reserves of mercury and antimony.
The Red Devil material, as received, was crushed to minus-½ inch, thoroughly mixed and sampled to obtain representative fractions for petrographic, spectrographs, and chemical analyses.
Physical Character
The ore consists primarily of ankerite, dolomite, muscovite and quartz, lesser amounts of stibnite, cinnabar, altered feldspar, clay minerals and chlorite, and small amounts of graphite dust and pyrite.
Petrographic study indicates that the stibnite and cinnabar are intimately intergrown. Both are essentially liberated from gangue in the plus-65-mesh fractions; however, small amounts of cinnabar and stibnite are locked with quartz and with each other even in the minus-200-mesh range.
Chemical Character
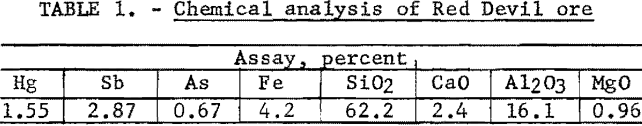
Partial chemical analysis of the ore is shown in table 1.
A semiquantitative petrographic analysis showed the presence and approximate amounts of the metals listed in table 2. Other elements, if present, are in quantities less than the minimum detectable by the routine method employed.

Procedure
A 5-ton sample of ore was treated by standard flotation techniques to produce a bulk mercury-antimony concentrate for subsequent furnace tests.
Ore was crushed to minus-½-inch and delivered by constant-weight feeder to the wet grinding unit, which comprised a 24- by 8-inch conical ball mill in closed circuit with an 18- by 48-inch spiral-type countercurrent classifier. The feed rate averaged 100 pounds per hour. The pulp density of the classifier overflow was maintained between 25 to 30 percent. Periodic screen analysis of the overflow showed that the ore delivered to the flotation circuit averaged 90 percent minus-100-mesh. The pulp was conditioned with promoter and collector in two 12- by 18-inch agitators in series. The sulfide minerals were floated and cleaned twice in standard 8- by 8-inch subaerated flotation cells (fig. 1).
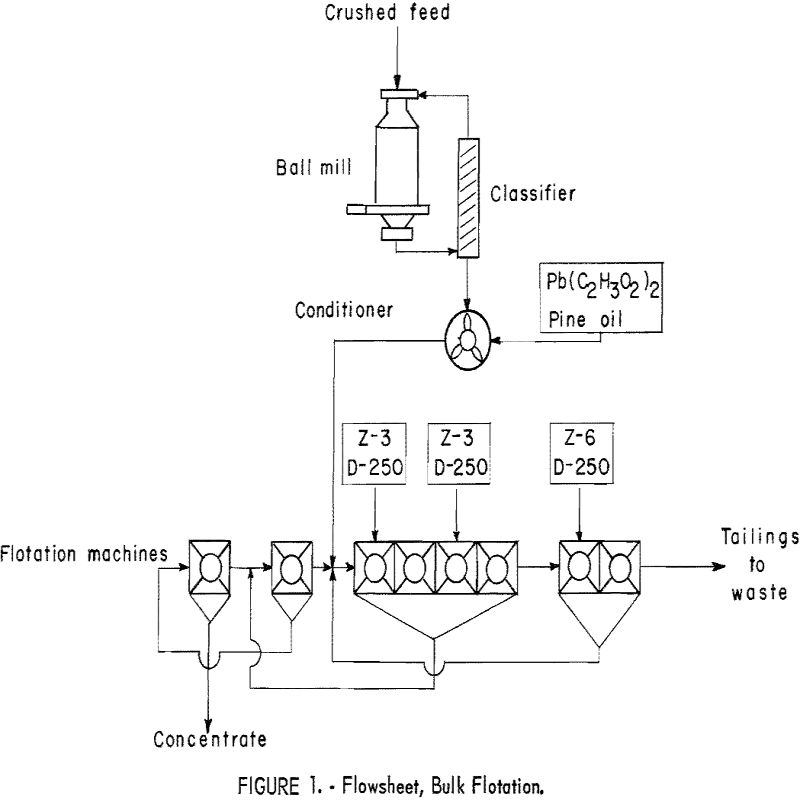
Additional collector was stage fed at various points in the circuit. Several collectors were tried, but potassium ethyl xanthate and potassium pentasol xanthate were used throughout most of the investigation. Lead acetate was used as a promoter for mercury; adding over 1-½ pounds per ton of feed, however, necessitated larger additions of frother. Water-soluble frother (D-250) was used for the primary frothing agent, supplemented by additions of pine oil or coal-tar creosote. Previous batch-flotation tests had indicated that adding 1 to 1-½ pounds of sodium cyanide to the conditioner would increase the grade of the concentrate. This was not verified by the results of continuous tests; thus cyanide was not used during most of the operation.
Flotation reagents were fed in dilute solution with a feeder developed at Washington State College (fig. 2). Essentially it is an electrolytic cell with
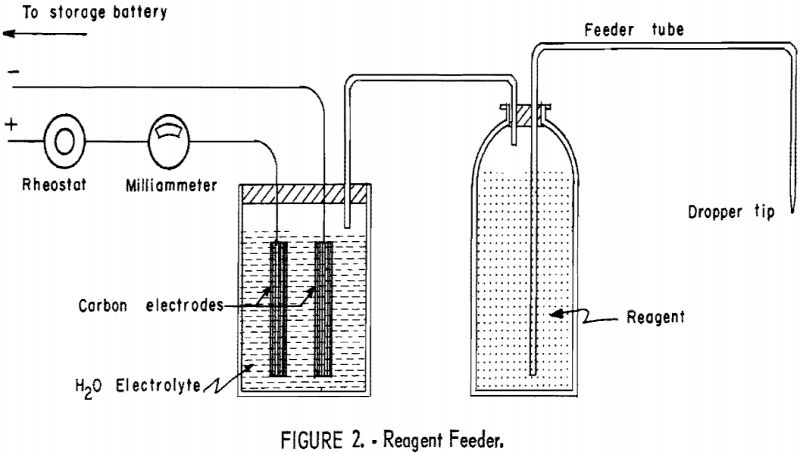
carbon electrodes and acidified water electrolyte. Oxygen and hydrogen are produced by decomposition of the water in direct proportion to the amount of current supplied to the cell. The gases, in turn, create a pressure in the reagent vessel and force an equivalent volume of reagent through the feeder tube. This feeder allowed positive control of reagents and exact duplication of quantities even after long periods of nonoperation, provided variations in room temperature were not excessive.
Timed samples of flotation feed, tailing, and concentrate were taken at regular intervals and composited for analysis for each 8-hour shift. Recoveries were calculated from the formula:
R = 100 c (h – t)/h (c – t)
where
R = percent recovery,
c = assay of concentrate,
h = assay of feed, and
t = assay of tailing.
The final concentrate was dewatered, air-dried, and thoroughly mixed to obtain representative portions for subsequent furnace tests.
Results
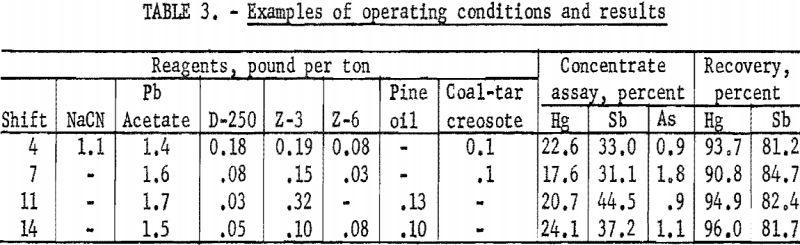
Because of minor mechanical difficulties and experimentation with flotation reagents, the results obtained varied from day to day. Recoveries ranged from 88.2 to 97.5 percent of the mercury and 76.1 to 86.1 percent of the antimony. The grade of concentrate varied from 16.9 to 27.4 percent Hg and 31.1 to 37.7 percent Sb. Operating conditions and results obtained from four typical shifts are shown in table 3.
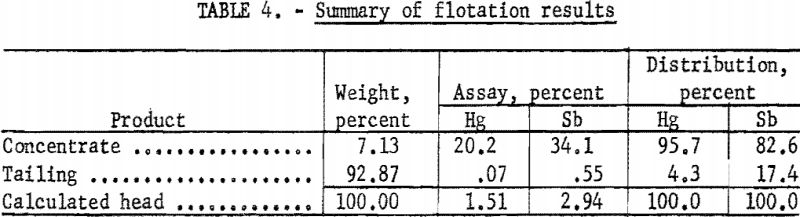
A summary of the weighted averages of the individual-shift data (see table 4) indicates an average recovery of 95.7 percent of the mercury and 82.6 percent of the antimony in a concentrate that assayed 20.2 percent Hg and 30.1 percent Sb. Actual analysis of the composite concentrate, used as feed for the roasting tests, showed 20.4 percent Hg and 34.6 percent Sb (table 5). The particle-size distribution of the composited concentrate is shown in table 6.
A petrographic study of samples of the flotation tailing showed that the bulk of the antimony was present as fine-grained stibnite intimately interlocked with quartz.
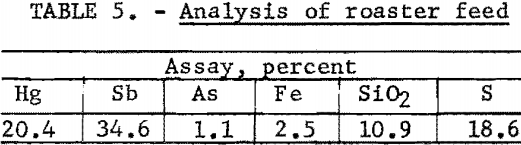
Extraction of Mercury from Cinnabar-Stibnite Concentrate
Mercury has only slight affinity for oxygen; hence roasting cinnabar in the presence of air proceeds according to the following reaction:
HgS + O2 = Hg + SO2.
Although the reaction starts at approximately 250° C., the reaction rate is relatively slow below the boiling point of mercury (357° C.).
Oxidation roasting of antimony sulfide forms volatile trioxide or nonvolatile tetroxide, depending on the temperature and amount of excess air. At slightly above 350° C., with a limited supply of air, the trioxide is formed:
2 Sb2O3 + 9 O2 = 2 Sb2O3 + 6 SO2.
With excess air, constant rabbling, and a temperature maintained at about 500° C., conditions are favorable for forming tetroxide:
Sb2S3 + 5O2 = Sb2O4 + 3 SO2.
Temperature control is important: With limited air, stibnite will fuse or liquate at about 550° C.; with excess air, a fusible mixture of sulfide and oxide (known as antimony glass) is formed at a slightly lower temperature.
Previous testing indicated that low-temperature roasting of material containing both cinnabar and stibnite favors oxidation of mercury sulfide before antimony sulfide; with a limited supply of air, most of the mercury can be volatilized while the bulk of the antimony remains in the calcine. Because of the narrow limits of temperature and air supply necessary for selective roasting of mercury-antimony concentrate, fluidized-bed roasting was adopted for this phase of the investigation. This process, noted for the ease with which temperature and atmosphere can be controlled, has found widespread use in the metallurgical field during the last 20 years.
Fluidization, basically, is the suspension of solid particles in a stream of gas moving upward through a perforated constriction plate. Just enough gas velocity is maintained to keep the solids in turbulent agitation throughout a zone of controllable height. The result is a fluid bed of solids with behavior similar to that of a liquid – it will seek its own level, overflow weirs or transfer pipes, and exert hydrostatic head so that differential pressure between points of various depths in the bed may be measured. Because of the violent movement of the particles, almost complete uniformity of chemical composition and particle size is maintained throughout the bed.
Heat may be supplied by the chemical reaction between the solids and the gas, or it may be entirely or partly obtained by burning gaseous, liquid, or solid fuels either inside or outside the reactor. Heat transfer between the solid particles and the gas film that surrounds them is so rapid that the temperature of the bed can be controlled automatically (with an accuracy of 10° to 20° F.) by injecting water from thermostatically actuated valves. Instrument control can also be applied to other phases of the operation. The flow of gas for fluidization can be metered accurately; gases can be automatically mixed, if necessary, to obtain a gas of predetermined composition.
The material to be treated must be crushed to a size suitable for fluidization; this size, dependent on the specific gravity of the material, is considered to be minus-14-mesh or finer. Dry feed may be introduced to the reactor with a screw-type feeder. If the material to be roasted is sticky or requires predrying, it can be fed by pump as a thick wet slurry.
Gas velocity through the voids in the fluid bed is much higher than in the space above the bed. Thus, the top of the agitated mass, although irregular, has a definite line of demarcation from the gas above. Most particles drop back into the bed and leave the reactor by overflowing the discharge pipe. The finer particles, however, are entrained with the upward-moving gas stream and carried out the upper end of the reactor. The dust solids are removed from the exit gas with cyclones, electrostatic precipitators, scrubbers, or combinations of such gas-cleaning units. The amount of carryover varies with the particle size of the material and with the velocity of the gas; it usually is considered to be greater than the dusting in multiple-hearth roasting but less than that in flash roasting and may be 20 to 40 percent of the total reactor product. In most instances there is little significant difference in chemical composition and degree of roast between the overflow product and the carryover; thus, the products can be combined, and the amount of entrainment is considered to be unimportant.
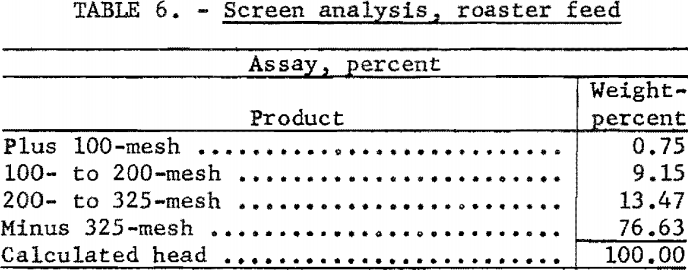
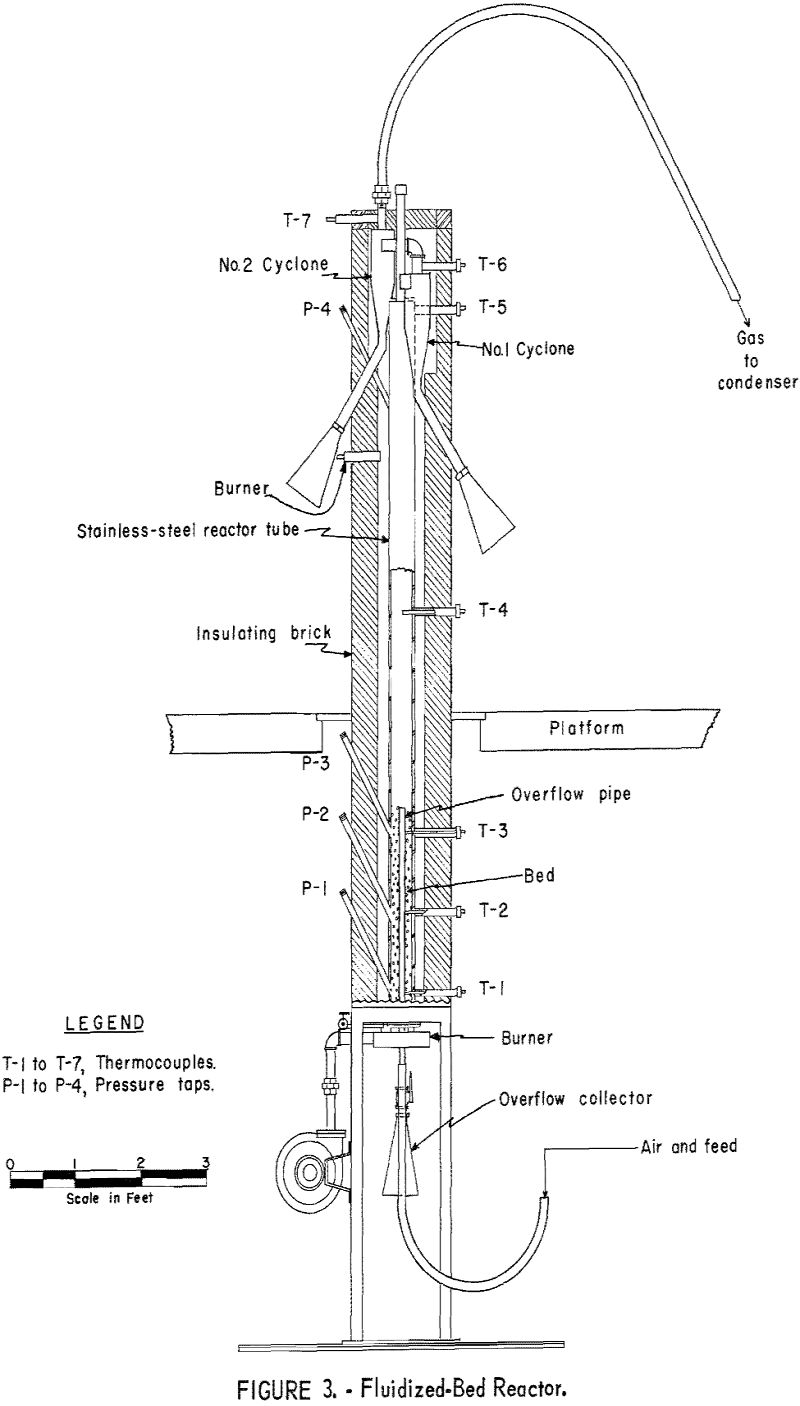
For this investigation a standard 4-inch laboratory reactor was employed (fig. 3). Concentrate was introduced to the system by a fluidizing gas stream, fed by a screw-type feeder driven by a variable-speed motor. Exit gases were passed through two 4-inch-diameter dust-collecting cyclones in series before entering the condensing unit. The condenser was a stainless-steel box, 1 by 7 by 4 feet in height, divided by baffles into 7 compartments (fig. 4). Gases entering the condenser traveled about 25 feet and reversed direction 7 times before discharging from the top of the last compartment. Although water sprays were available in 6 of the compartments, experience showed that condensation of mercury was complete when only 2 sprays were used.
During the first test the condenser gases were discharged near the intake of the standard centrifugal fume-hood fan that exhausted the gases from the building. Later, the fan was connected directly to the condenser discharge pipe which eliminated back pressure caused by the condenser system and resulted in more efficient operation of the dust-collecting cyclones. By adjustment of a damper in a bypass pipe, enough suction was applied to the condenser discharge to maintain the condenser system under slight vacuum. Care was taken, however, that excessive suction did not upset the equilibrium of the fluidized bed.
Because of the volatility of mercury, even at room temperatures mercury vapor is present in the air wherever the metal is worked. Mercurialism or “mercury poisoning,” an illness contracted by inhalation of the vapor, was known by the goldsmiths and alchemists of the Middle Ages.
To maintain adequate health and safety standards the atmosphere of the laboratory was constantly monitored with a photoelectric detector. Operating employees were required to wear mercury-absorbent respirators and to follow rigid personal-cleanliness practices.
Preliminary Roasting Tests
Several series of batch and short continuous tests were made in the reactor to observe the effects of temperature variation, gas composition, feed rate, and other factors influencing the selective extraction of mercury from cinnabar-stibnite concentrate. A study of the data obtained resulted in the general conclusions discussed below:
Temperature
As was expected, the best extraction of mercury from the concentrate was obtained when the fluidizing bed was maintained at 480° to 520° C. Tests run with bed temperatures below 450° C. yielded calcines of relatively high mercury content. At about 520° C. “freezing” or defluidization occurred. Freezing is caused when the
solids become sticky because of fusion or sintering or when excessive amounts of sulfates or products of lower melting points are formed. A cursory examination of the frozen material after cooling showed that both liquated stibnite and antimony glass appeared to be present.
Automatic temperature control was not employed in the laboratory. After optimum gas composition and velocity were developed, no difficulty was experienced in manually maintaining a temperature of 485° to 490° C. throughout the bulk of the bed. The extreme bottom of the bed, continually cooled by feed and gas, was 15° to 20° lower. The reactor zone above the bed was held at 500° to 520° C. The temperature of the gases passing into the dust cyclones was 440° to 465° C. The gases cooled to about 85° C. before they entered the condenser unit; they were discharged at 18° C.
Retention Time
Retention time of the concentrate in the roasting zone is a factor of both rate of feed and depth of the fluidizing bed. Bed depth for most tests was held at 30 inches. Uniformity of temperature was more difficult to maintain with shallower beds; deeper beds increased dust carryover.
In commercial practice feed usually is introduced to the reactor bed with a screw-type feeder if dry or is fed as a wet slurry with a pump. In the laboratory unit the ore or concentrate is conveyed to the reactor by the fluidizing-gas stream, thus limiting the feed rate to the amount readily carried by the gas. Consequently, feed-rate variation to control retention time in the laboratory equipment was limited accordingly. In addition, feed must be very dry, as only slight amounts of moisture cause the material to form lumps that clog the feed line. Although feed rates as high as 200 grams per minute were used successfully on some tests, it was found that more uniform operation was obtained when the feed rate was held at approximately 130 grams per minute.
With a 30-inch bed and the feed rate fixed at 130 grams per minute, retention time of ore particles in the roasting zone was calculated to be 30 minutes. This may be much longer than actually is required for extracting the mercury. Experimentation with a unit allowing a more flexible feed variation would be required to establish the minimum retention time accurately.
Space Rate
The velocity or space rate of the fluidizing gas may vary for different feeds from 0.3 to 1.5 feet per second. The minimum velocity that will properly fluidize the bed usually is accepted, because space rates above the minimum increases the amount of material carried out as dust.
Because the concentrate used in this study was extremely fine, fluidization of the bed was accomplished at a low space rate – 0.50 foot per second. During the course of the investigation, space rates as high as 0.81 foot per second were tried. Within this range of velocities, no difference in operating conditions or in results was noted other than the tendency toward increased dusting as gas velocity increased.
Gas Composition
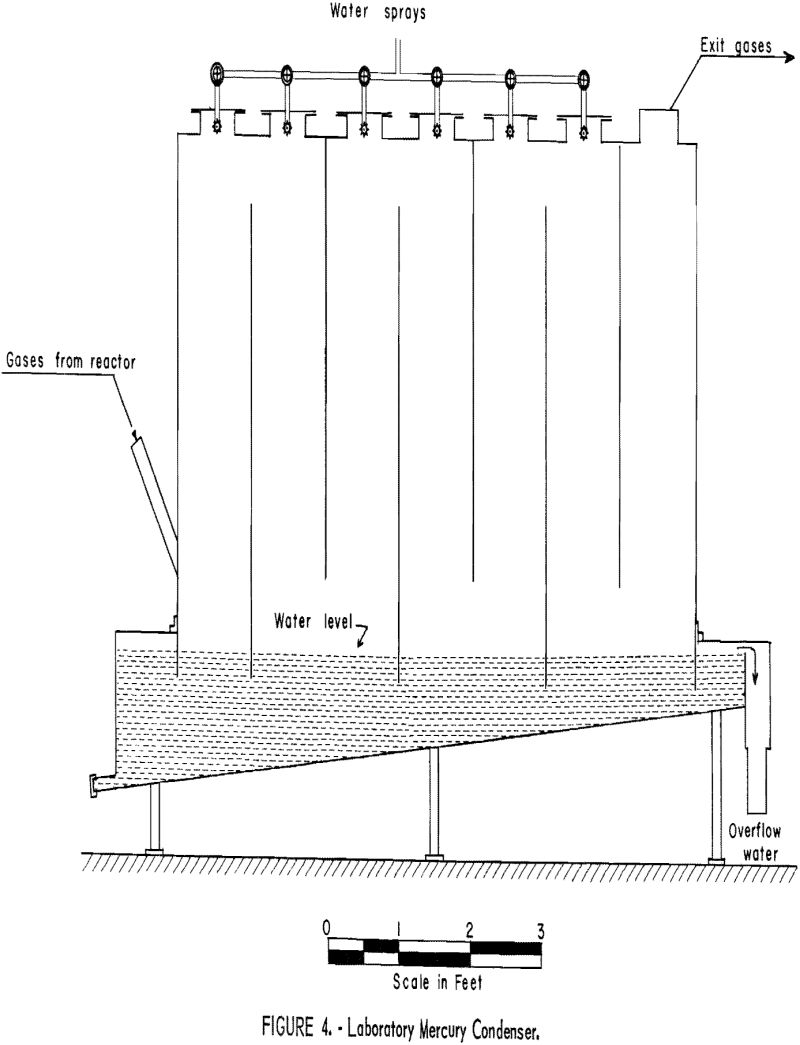
Attempts to use air as the fluidizing medium were unsuccessful; the quantity required to fluidize the ore bed introduced enough excess oxygen to cause the sulfides to burn rapidly. This highly exothermic reaction quickly raised the bed temperature above the softening point of stibnite; consequently, fluidization ceased. Mixture of propane and air that burned inside the reactor likewise were unsatisfactory. Because the ignition temperature of propane in air is approximately 500° C, ignition took place immediately above the surface of the bed. This caused incipient fusion of material at the bed surface and immediate freezing.
Preliminary tests were run using mixtures of helium and air as the fluidizing medium. Feed and gas rates were controlled to allow only slight excess of oxygen above the theoretical amount necessary for reaction with the mercury sulfide. The averages shown in table 7 were computed from the results of three tests using gases of low oxygen content.
In fluidized-bed roasting practice, the carryover solids usually are considered to be substantially the same as the overflow calcine. In the laboratory, however, a significant difference existed in the mercury content of the products. For example, the preliminary tests showed that an overflow product low in mercury could be made with a gas having an oxygen content only slightly in excess of the stoichiometric amount.

Petrographic study of a portion of the overflow assaying 0.19 percent Hg indicated that the mercury was present as minute cinnabar particles completely included in grains of stibnite. This indicated that overflow products appreciably lower than 0.2 percent Hg would not be obtained without oxidation of much of the antimony sulfide. A study of the dust collected in the first cyclone, however, showed the presence of fine free particles of cinnabar. It is believed that this was not unreacted feed but rather artificial cinnabar created by the recombination of mercury vapor and sulfur-laden gas. The product of the second cyclone was a mixture of cinnabar, mercury globules, and unidentifiable dust particles. This material, similar to the sludge collected in the condenser, assayed as high as 60 percent Hg in some tests.
It was decided that the dust from the second cyclone and the residue from the condenser should be returned to the reactor for re-treatment. The dust collected in the No. 1 cyclone, however, represented too much weight (approximately 20 percent of the total feed) to be recycled economically yet contained too much mercury to be rejected with the overflow product. Efforts were directed, therefore, toward lowering the mercury content of this product to approach that of the overflow.
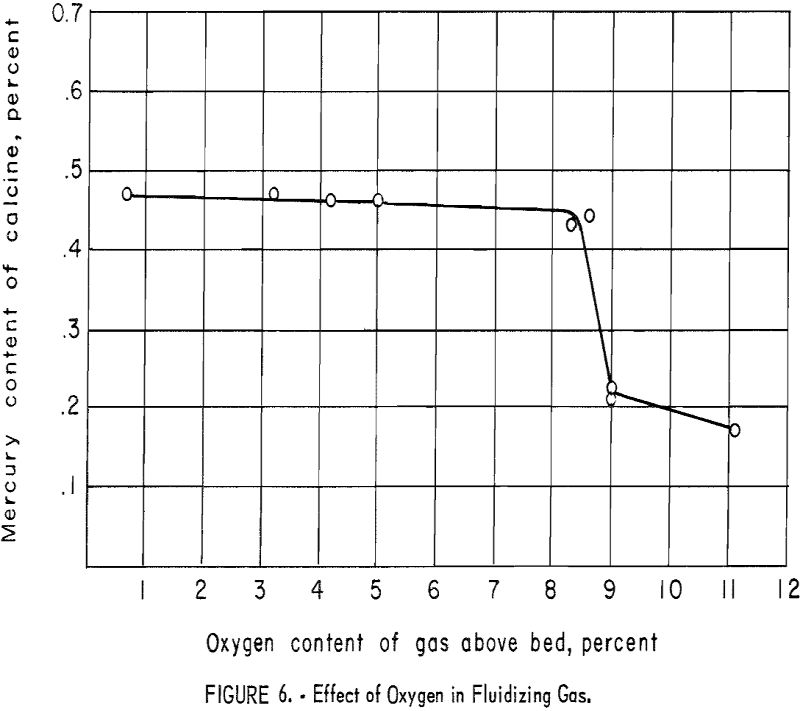
The effect of increased oxygen content of the fluidizing gas was investigated. Helium-air and nitrogen-air mixtures were used successfully. In later tests a pre-ignition chamber or “Dutch oven” was employed to reduce costs (fig. 5). Metered amounts of propane and air were burned to yield combustion gases of the desired composition. For comparison of results, the oxygen content of the gases above the bed were determined by means of an Orsat gas analyzer. The relationship of mercury content of the calcine (overflow plus first cyclone product) to the oxygen content of the gases above the bed is shown graphically in figure 6. It will be noted that no significant change in the mercury content of the calcine was affected until the oxygen content of the gases exceeded approximately 9 percent.
Increasing the oxygen content to 11 percent did not remove an appreciable additional amount of mercury. Higher oxygen contents of the fluidizing gas were not investigated, because previous testing had shown that the presence of an overabundant supply of oxygen, supplied by air undiluted by an inert gas, resulted in rapid burning of the sulfides in the ore and subsequent freezing of the bed.
Approximately twice as much antimony sulfide was oxidized in the “9-percent oxygen” tests as in those that employed little excess oxygen.
Addition of Air to Exit Gases
Although additional oxygen in the fluidizing gas successfully lowered the mercury content in the No. 1 cyclone dust, little effect was noted in the products of the second cyclone and the condenser. Introducing air to the dust-laden gas stream was investigated as a means of reducing the mercury sulfide content of the dust. Adding air above the fluidizing bed immediately oxidized the fine sulfide particles in the gas stream. The reaction was exothermic; not only was the heat transferred to the bed to cause softening and freezing, but a layer of sinter was formed on the walls of the reactor above the bed. Introducing air between Nos. 1 and 2 cyclones produced a less violent reaction, because approximately 70 percent of the sulfides in the gas stream were removed by the first cyclone. The mercury sulfide was completely oxidized; no trace of cinnabar was found in either the second cyclone product or condenser residue in any of the subsequent tests. In addition, the increased gas velocity resulting from the admission of air apparently swept out much of the fine metallic mercury that previously had collected in the second cyclone. The dust product previously had assayed up to 60 percent Hg and represented 12 to 15 percent of the total feed. After the change, the material assayed only 3 to 5 percent Hg and represented only about 6 percent of the feed.
Several tests were made in which the oxygen content of the fluidizing gas above the bed was maintained at about 9 percent and additional air was introduced to the exit gases of the first cyclone. Results shown in table 8 are typical of those obtained from these tests.
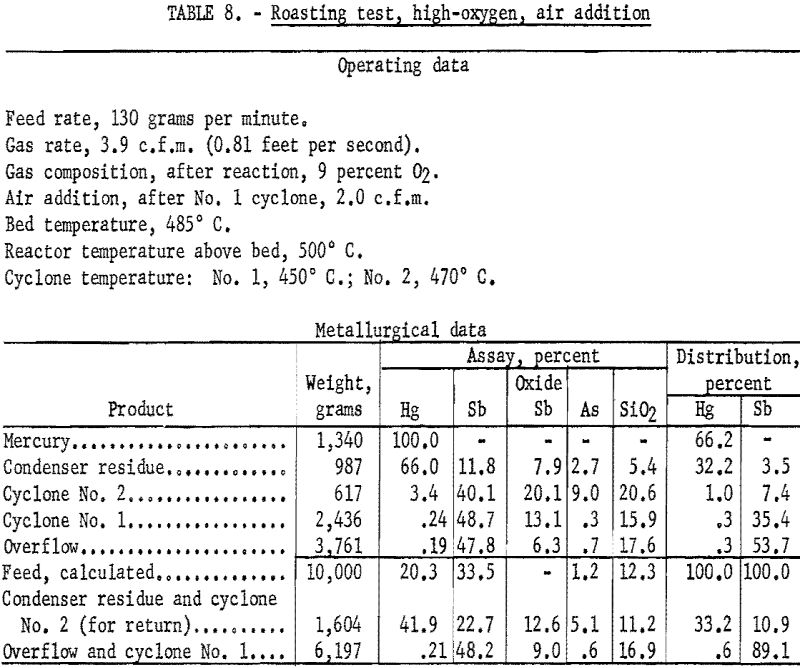
The practice of introducing air to oxidize the dust in the gas stream had one definitely detrimental effect. Study of data from tests using the additional air technique indicated that 65 to 85 percent of the antimony sulfide escaping the first cyclone was converted to the trioxide, Sb2O3. It is assumed that at least as high a proportion of the escaping arsenic also was oxidized. Thus, although the second cyclone effected the removal of about two-thirds of the antimony and arsenic passing the first cyclone, considerable amounts of the oxides of these metals reached the condenser system; the ability of these oxides to “sicken” mercury and hamper its coalescence is well known. Had equipment been available an electrical precipitation dust collector would have been tried as a substitute for the second cyclone in an effort to remove more of the dust before it entered the condenser.
Condenser Products
The formation of condenser “soot” always has been a problem when producing mercury. This soot consists of dust that has escaped the dust collectors, sulfur precipitated from the gas stream, mercury, water, and precipitates of volatile constituents of the ore, such as arsenic and antimony, The arsenic and antimony oxides, with the dust and sulfur, tend to coat fine globules of mercury and to prevent their coalescence.
Most of the mercury caught in the laboratory condenser was present as soot. This may have been caused by the unusual amount of antimony in the roaster feed, improper functioning of the second cyclone, or the design of the condenser. In addition, the necessity of cleaning the condenser after each short run allowed no buildup of sludge, a condition which would be conducive to coalescence.
Treating the soot by filtration through canvas removed approximately 60 percent of the total mercury as clean metal. The filter cake was pulped with water and lime to recover an additional 10 percent of the mercury. The residue of the soot, representing 7 to 10 percent of the original feed and containing 20 to 30 percent of the total mercury, was dried, sampled, and assayed. It was assumed that this product, combined with dust from the second cyclone, could be returned to the roaster for re-treatment.
All the metallic mercury was cleaned in an “oxifier” and filtered to remove floating impurities such as dust and base-metal oxides. The resulting product contained less than 0.005 percent residual impurities and met all chemical and appearance specifications for virgin mercury metal.
Condenser Losses
Periodic checks were made of the mercury content of the gases discharged from the condenser, using a mercury-vapor-detection meter. Under no conditions did the off-gases exceed 0.2 milligram of Hg per cubic meter; this represented only about 0.002 percent of the total mercury in the feed.
It is believed that quicksilver lost by solution of mercury salts in the condenser overflow water was almost negligible. Samples of water taken during the early tests did not show significant mercury content. Later tests (high oxygen) indicated that the overflow water carried some fine mercury globules, probably owing to increased “sickening” caused by the formation of additional antimony oxide. In practice, almost all the mechanically held metal would be recovered by passing the overflow water through baffled launders and settling tanks.
Loss of mercury by absorption or penetration in the walls of the condenser was precluded by the use of stainless steel. This loss, once a major item in plants using porous tile or brickwork condensing systems, largely has been eliminated by constructing condensers from material impervious to mercury vapor.
Calculations of metallurgical balances for the short batch tests were made without allowing for condenser losses. In calculating results of the longer continuous tests, however, these data were included.
Continuous-Roasting Test
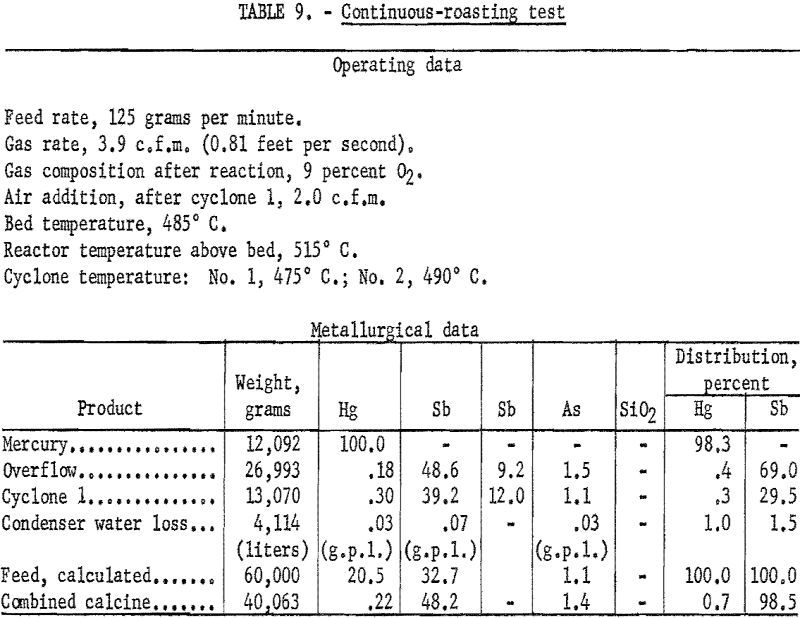
A continuous roasting test was made, based upon the best operating techniques established by the short batch tests. The product of the second cyclone was cooled in the absence of air and returned to the feed. Return of the condenser residue was simulated by adding condenser residue from previous tests to the feed. The final products, therefore, were mercury metal and a calcine consisting of the combined overflow and No. 1 cyclone products. Table 9 shows the operating data and the metallurgical results obtained.
Results and Observations
Adding the dust product from cyclone 2 and the condenser residue to the feed raised the mercury content of the feed to approximately 22 percent. Treating the composited feed, therefore, required the use of a fluidizing gas richer in oxygen than treating the flotation concentrate alone. As shown in table 9, more than 98 percent of the mercury in the composited feed was recovered in the metallic form. One percent of the calculated loss was that mercury carried out of the circuit by the condenser overflow water. With proper launder and thickening equipment, at least part of this loss could be recovered; thus extractions up to 99 percent in plant production might be expected. More than 98 percent of the antimony in the roaster feed reported in the calcine at a grade of 48.2 percent Sb, 1.4 percent As, and 0.22 percent Hg. This product might serve as the basis for producing a marketable antimony byproduct. Laboratory investigations to develop an economic treatment for producing an antimony product from the fluidized-bed calcine will be the subject of another paper.
Laboratory tests indicated that an overall mercury recovery of about 94 percent can be made by bulk flotation of cinnabar-stibnite ore, followed by fluidized-bed roasting of the concentrate. Further, the tests indicated that the treatment is mechanically and metallurgically feasible. Incorporating slurry feed equipment and the automatic temperature and feed-rate controls would eliminate the necessity for drying the concentrate and simplify many of the operating troubles encountered with the experimental roaster.
Extraction of Mercury from Cinnabar Concentrate
Preliminary tests were made to investigate the application of fluidized-bed roasting for treating antimony-free cinnabar concentrate. Tests were conducted on two feeds prepared to simulate flotation concentrate of different grades. Partial chemical analyses of the feeds are shown in table 10.

Preliminary Roasting Tests
Without the presence of antimony in the feed it is unnecessary to maintain close control of the roasting temperature. In the laboratory tests air was used for fluidizing; the temperature of the bed was maintained at about 520° C. in most tests but approached 570° C. in others. Admitting air to the exit gases of No. 1 cyclone had little effect if the volume of the fluidizing gas was high enough. Because the feed had lower specific gravity than the mercury-antimony concentrate, lower space rates could be employed. Enough dust entered the condenser so that all the mercury was present as soot. The condenser product, however, responded well to treatment so that the residue after removal of coalesced mercury rarely exceeded 5 percent of the feed.
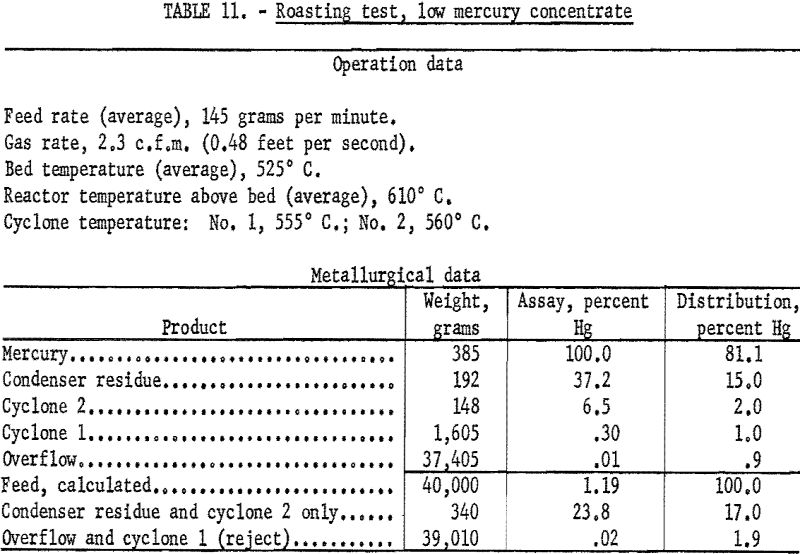
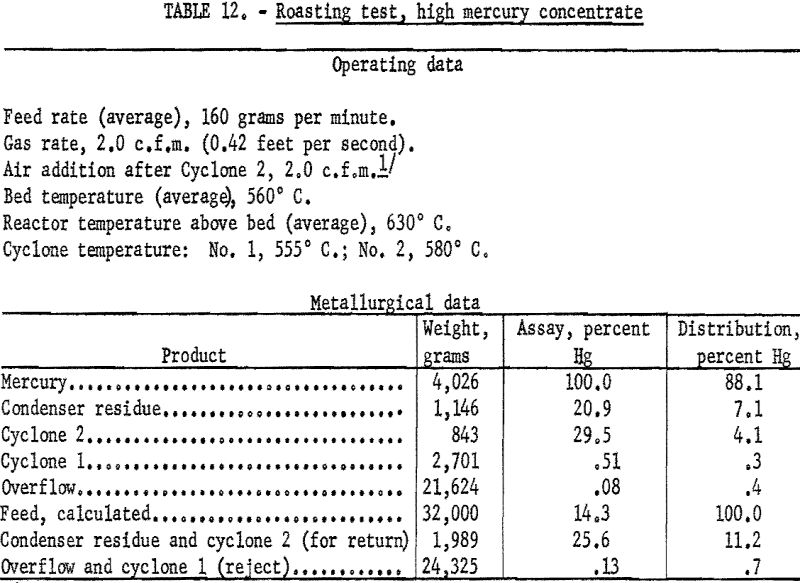
Data from three tests were composited (table 11) to show average results of the treatment of the 1.1 percent Hg feed. Similarly, average results from the treatment of the 14-percent Hg feed are shown in table 12. Both high and low mercury products were amenable to treatment by fluidized roasting for an indicated extraction of more than 98 percent of the mercury. This continuous treatment, adaptable to any scale of operation, conceivably could completely replace retorting as a treatment for mercury concentrate.