Table of Contents
The paper is based on laboratory and pilot plant investigations which led to the development of a series of processes for production of high purity metals from nickel-copper-cobalt mattes of a wide range of opposition.
Matte Refining
The application of these processes to the refining of mixed base metal mattes is governed by several factors, some of which are discussed in greater detail later in this paper. Basic to any consideration of matte refining are the contents of iron and sulphur. In general, matte will have much loss iron and much less sulphur than concentrate, for an equivalent weight of nickel, copper or cobalt. The process of smelting to produce matte rejects a large proportion of the iron, and the sulphur associated with it. When producing a matte for refining by these processes, the iron content can advantageously be lowered to the minimum consistent with good recovery of base metals in the matte. During leaching, iron must be oxidized and this requires additional oxygen in the form of compressed air above that needed for leaching the valuable metals.
When iron and sulphur contents have been settled by the smelting conditions, the composition of the entire matte is defined for any given raw material. The following factors can then be considered:
a) The selection of either ammonia or acid leaching is influenced by local economic of the matte.
b) The purification steps, and their sequence, can now be outlined for the other metals present in the matte.
c) The selection of one of the two methods of recovering metallic nickel and cobalt from the purified solutions can be mine, the selection being chiefly influenced by the nickel/cobalt ratio in the purified solution.

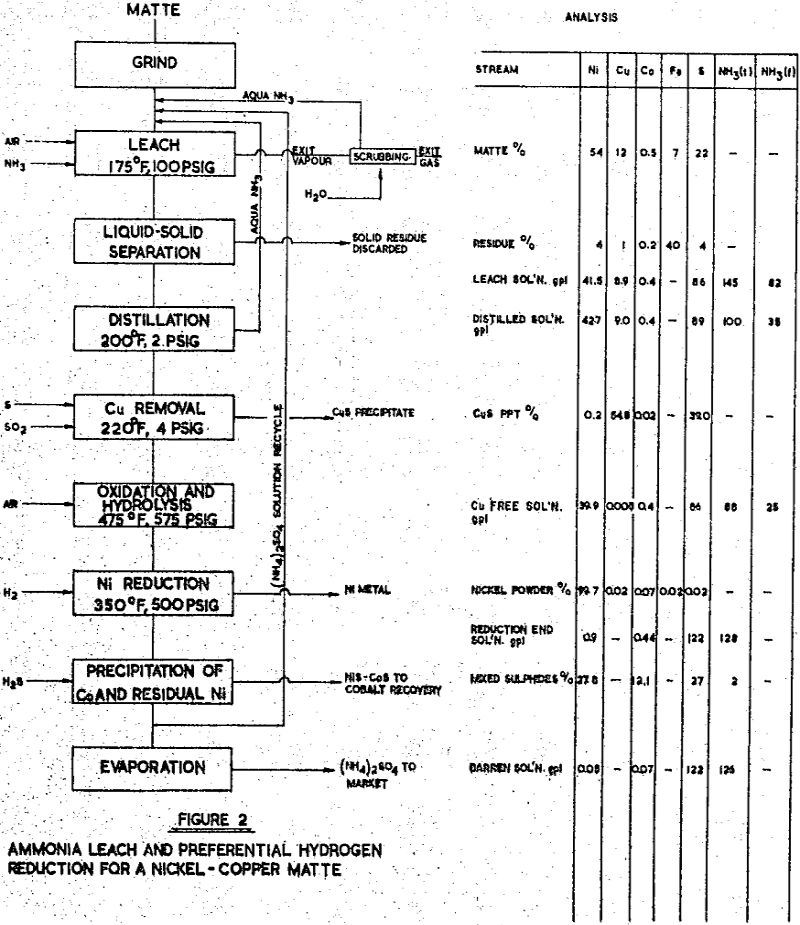
Due to the presence of metallics, grinding was conducted to a fineness of 1% plus 65 mesh, which produced 90% minus 200 mesh. With the low iron content, a low ammonia content was furnished in order to leach the metallics. Since the ‘free ammonia’ content of the leach solution was low, the temperature could be raised from 175°F to 200°F without unduly increasing the vapour pressure of the solution.


Matte of this general composition is encountered more frequently than the others in this series, due to the natural association of nickel and copper sulphide minerals in many parts of the world. Usually, a flotation separation may be made to provide a copper concentrate low in nickel, and the nickel concentrate then contains some copper. Of several mattes of this type that have been studied, one of the above analysis has been selected for discussion. The leaching of this matte was investigated in a series of laboratory tests, followed by a systematic study of operating conditions and controls in a continuous pilot plant campaign extending over a three month period.
The leach solution was distilled to remove the free ammonia content which was required for leaching, but which was in excess of the requirements for nickel reduction. The ammonia and water vapours were condensed and recycled to the leach feed.
Copper was removed from the solution by precipitation as a sulphide, using the sulphur dioxide – sulphur process developed by Sherritt Gordon. The copper product, at 55 – 60% Cu and 35 – 40% S was suitable for smelting in an adjacent smelter. The final purification for unsaturated sulphur, by high pressure oxidation and hydrolysis, was followed by preferential nickel reduction, as before. The nickel powder product assayed:
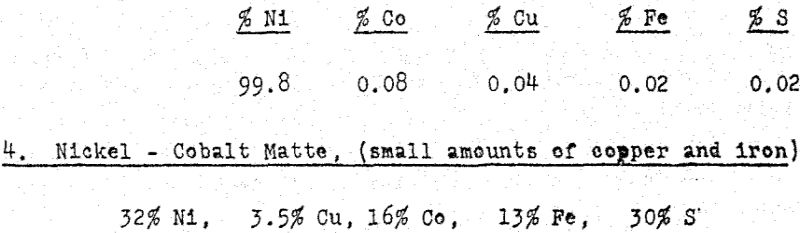
This matte differs from the preceding mattes, not only in the high cobalt content, but in its high sulphur content; metallics were largely absent, and sufficient sulphur is available to form sulphates of nickel, copper and cobalt.
Shipments of this matte were studied in laboratory tests and also in the continuous pilot plant. The flow diagram is shown in Figure 3. The laboratory tests showed a calcium content of the matte sufficiently high as to cause trouble with calcium sulphate in solution a direct leach of the matte produced a solution with 52 gpl; Ni, 26 gpl. Co and over 0.7 gpl, Ca, with calcium sulphate crystallizing from the solution.
Process Chemistry
The chemistry of leaching sulphidic materials in ammonia solutions with air has been discussed in detail in earlier publications. Nickel, copper and cobalt are oxidized and pass into solution as metal ammine sulphates, while iron is oxidized and remains as an insoluble hydrated oxide. Some of the sulphur may be incompletely oxidized to form unsaturated compounds such as thionates, thiosulphates and sulphamates.
The basic reactions that occur during ammonia leaching may be represented as follows:
NiS+ 2O2 + xNH3 → Ni (NH3)xSO4…………………………………………………………….(I)
FeS+ 2¼O2 + 1½H2O + 2NH3 → ½Fe2O3·H2O (NH4)2SO4………………………(II)
Ni + (NH4)2SO4 + ½O2 → Ni(NH3)2SO4+ H2O……………………………………….(III)
The reactions which take place in acid leaching are indicated by the following equations:
NiS+2O2 → NiSO4…………………………………………………………..(IV)
FeS + 2O2 → FeSO4………………………………………………………….(V)
Ni + H2SO4 + ½O2 → NiSO4 + H2O………………………………..(VI)
2FeSO4 + H2SO4 + ½O2 → Fe2(SO4)3 + H2O…………………………………..(VII)
Fe2 (SO4)3 + 2H2O → 2Fe(SO4) (OH) + H2SO4……………………………….(VIII)
There are a number of principal variables which affect the rate of leaching and the final extraction of the valuable metals from a given matte. In general, they apply to both ammonia and acid leaching.
- Screen analysis of leach feed: it has been found that material should be ground to pass 65 mesh for good leaching. In practice, all though 65 mesh means some 95% passing 100 mesh and 70 – 80% passing 200 mesh.
- Agitation: sufficient agitation must be supplied in the leaching vessels, to suspend the solid fraction in the leach solution, to disperse the air in fine bubbles throughout the slurry and to provide the movement necessary to remove concentrated leach solution away from the solid particles.
- Metals concentration: the practical limit for metal concentration in ammonia leach solutions, under normal conditions, has been determined as 50 gpl. of nickel copper concentration may be higher, whereas the limiting cobalt concentration is lower.
- Air flow: In laboratory tests, a cylinder of oxygen is connected to the vapour phase of the autoclave, and oxygen flow is on a demand basis. In commercial operation air is used as the source of oxygen.
- Pressure: as noted immediately above , the partial pressure of oxygen in the leaching vessel may be rate controlling in the early stages, but other factors superside it in the later stages.
- Temperature: the temperature at which ammonia leaching is operated is influenced by several factors. Increasing temperature will increase the rate of reaction, but will also decrease the solubility of ammonia and oxygen in water, will increase the vapour pressure of ammonia and water vapour and so decrease the oxygen partial pressure if the total pressure remains constant.
- Retention time: the time required to complete an ammonia leach is primarily a function of the leachability of the particular feed material, the temperature and the pressure.
- Reagent concentration: it has been shown elsewhere that the ammonia concentration during leaching has a marked effect on the rate of leaching and, under certain conditions, on the extraction obtained.
- Ammonium sulphate concentration: the pressure of ammonium and sulphate ions in ammonia leach solutions are necessary for tho rate of leaching to be acceptable, and usually there is sufficient sulphur in the feed material to provide a suitable concentration of sulphate.
- Calcium concentration: calcium is undesirable in this process for two reasons calcium sulphate becomes less soluble at high temperatures, and can precipitate during hydrogen reduction, with consequent high sulphur content of the metal powder.
Solution Purification
The nature and sequence of the steps required for solution purification depend upon the mature of the leach solution. Separation of the solution from the insoluble leach residue is always required; iron and copper are frequently encountered; unsaturated sulphur compounds such as sulphides and thiosulphate ions are also usual. In addition, a number of minor elements have been met. It will be noted that copper removal is classed as ‘purification’, inferring that copper is an impurity.
Iron removal: the solutions derived from leaching with ammonia – ammonium sulphate are iron free, and the leach slurry requires only liquid-solid separation. If the iron content of the feed matte is low, as in matte Nos. 1 and 3, direct filtering in pressure filters is indicated. A high iron matte, such as No. 2, will produce a large amount of residue — in this particular case, one ton of residue for one ton of matte — and thickening followed by vacuum filtration, or counter-current-decantation is preferred. Several of the new synthetic flocculants have proved advantageous in this service.
Precipitation of iron may be conducted on the leach slurry before liquid-solid separation, or on the leach solution after thickening er filtering. In tho latter case, If the Iron precipitate contains significant amounts of copper or other metals, it may be returned to the leach feed. Filtration of iron precipitate is not troublesome; the use of vacuum leaf or pressure plate and frame filters usually precoated has been demonstrated in several pilot plant campaigns. Multifilament nylon or conventional cotton, canvas media is used.
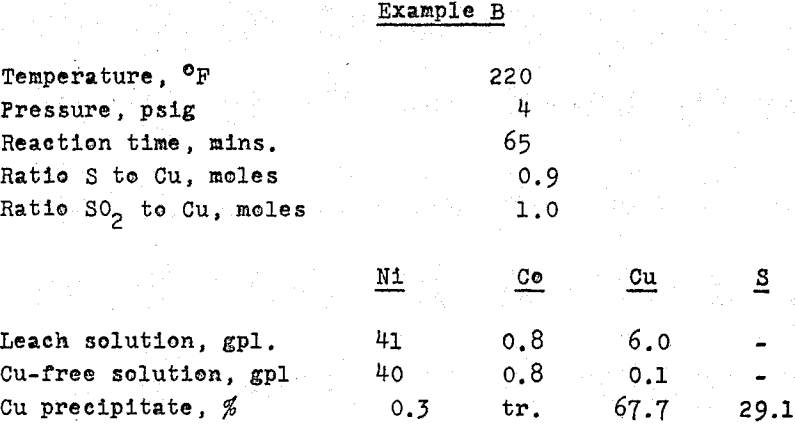
Copper removal: the ‘iron-free’ solution usually contains copper, with the concentration ranging from traces to several grams per litre. The following table shows the analyses of some of the solutions from matte investigations;

Where substantial amounts of copper are to be removed from an ammonia leach solution, as No. 3, precipitation as sulphide may be carried out following a technique developed in the Research Laboratories of Sherritt Gordon.

Metals Recovery
The solution produced by the purification steps contains nickel, cobalt, ammonium and sulphate ions; detrimental impurities have been removed, although innocuous elements, such as zinc or manganese in small amounts, may be present. The recovery of nickel and cobalt from this solution by reduction with hydrogen is now feasible; the procedures used depend upon the relative concentrations of nickel and cobalt in solution. If the nickel content is substantially greater than the cobalt content, direct reduction of nickel by the ‘Preferential Reduction’ method is indicated, whether the solutions be from ammonia, or from acid leaching. If the nickel content is less than the cobalt content, separation of the two metals prior to reduction is indicates, using the ‘Soluble Cobaltic Ammine’ method. When nickel and cobalt are approximately equal, either method may well be used the selection depending upon local conditions.
The metal powders produced by hydrogen reduction are recovered by filtering and are washed and dried. Screen analysis of the powder will normally be between 100 mesh and 10 microns, and is controllable to some degree by the characteristics of the reduction vessel and its operating cycle. The powders can be briquetted if this is desirable. Chemical analyses of representative powders have been shown in the earlier examples.