Table of Contents
More than 95 percent of the total tin in Bolivian mine-run ores can be volatilized and recovered as tin chlorides at temperatures ranging from 520° to 565° C. Equally high percentage recoveries of tin can be obtained from low-grade Bolivian concentrates at temperatures of 565° to 600° C. Minus 35-mesh ores and concentrates can be chloridized readily.
Cassiterite in mine-run ores and low-grade concentrates can be reduced and chloridized by contact with mixtures of hydrogen and hydrogen chloride containing 15 to 85 percent hydrogen chloride. The optimum percentage of hydrogen chloride in the gas mixture depends largely upon the quantity of tin in the ore or concentrate. Pyrite, particularly in mine-run ores, also affects the ratio of hydrogen to hydrogen chloride, as substantial amounts of hydrogen chloride are consumed in chloridizing iron during the thermal decomposition of pyrite.
A rapid flow of the gaseous mixture of hydrogen and hydrogen chloride is needed to sweep tin chlorides, which are collected readily, from the reaction zone. Of course, in a continuous process the circulating gases would require some enrichment.
Approximately the same time of treatment was required for the mine-run ores and the low-grade concentrates. Although the relation of the yield of tin to the time of treatment and the rate of gas flow was not established experimentally, a trend was observed which indicated that recovery of tin chlorides by volatilization could be equaled in less time with an increased rate of gas flow.
In comparison with the current metallurgical methods for recovering tin, certain advantages in the chloride volitization method are indicated:
- (1) Tin can be recovered from mine-run ores, thus eliminating the need for mineral dressing and the attendant losses of tin in slimes.
- (2) Substantially lower temperatures are required, although this economy may be somewhat offset by the need to heat larger amounts of material to obtain equal quantities of tin. In some cases, the use of a low-grade concentrate would be helpful in compensating for the increased cost, unless too much tin might be lost in mineral dressing.
- (3) Losses of tin in slags, flue dusts, and hardhead (an alloy of tin and iron) are avoided.
- (4) As volatilized tin chlorides contain less than 0.5 percent iron and only traces of arsenic and sulfur, refined tin can be obtained readily by electrolytic methods.
Tin could be recovered in the vicinity of tin deposits. Bolivia has deposits of pyrite and salt for producing hydrogen chloride, hydrocarbon gases for cracking, and petroleum fuel (or possible hydroelectric power) as a source of heat energy.
The purpose of this research by the Federal Bureau of Mines was to investigate the recovery of tin from mine-run ores and low-grade concentrates. Over 95 percent of the total tin in such materials was volatilized to tin chlorides at temperatures ranging from 520° to 565° C. Gaseous mixtures of hydrogen and hydrogen chloride were used for reducing cassiterite to stannous oxide and for converting this compound to tin chlorides. The chlorides were swept from the reaction zone of an oscillating kiln by a rapid flow of reactant gases and collected in a condensing system.
Losses of tin that occur in current metallurgical processing were prevented. Approximately the same time of treatment was required for minus 35-mesh mine-run ores and low-grade concentrates. In a continuous process, the circulating gases would require some enrichment.
This report is one of a series describing experimental work being conducted at the Bureau’s Rolla Metallurgy Research Center for the recovery of tin. In this work a low-temperature chloride volatilization process is used to remove tin from slimes, concentrates, and mine-run ores. Although it has been stated that primary tin is not in short supply/improved methods of recovery are needed in the interest of conservation, as the known tin resources of the world are less than those of other strategic metals, such as copper, chromium, nickel, and tungsten.
Current methods for concentrating tin ores and recovering the metal by smelting permit losses of tin in tailings, slags, and flue dusts. Temperatures of 450° to 600° C. are adequate for volatilizing tin as chlorides, whereas temperatures above 1,000° C. are required for smelting. Furthermore, gravity concentration, which at present is reported to recover only 50 per-cent of the total tin from Bolivian ores, results in substantial tin losses. The failure of gravity concentration to recover more of the tin is due to the fineness of cassiterite grains in the ores. Moreover, requirements established by smelters are for a high-grade concentrate of 60 to 70 percent tin.
Conditions in Bolivia are not favorable for tin smelting, and other methods are needed for winning tin. Supplies of pyrite are available, and this mineral could be used as a source of heat and sulfur for volatilizing tin as a sulfide from tin-bearing concentrates. Research on this method of recovering tin is reported by the Bureau of Mines and is covered by several patents.11 Temperature requirements range from 800° to 1,000° C.
Bolivia is reported to have petroleum for fuel, deposits of salt and pyrite, and possibilities for developing hydroelectric power. These assets are favorable for a low-temperature chloride volatilization process. Hydro-electric power and petroleum could be used as a source of heat, salt and sulfuric acid could be reacted to produce hydrogen chloride, and hydrocarbon gases could be cracked to obtain hydrogen. To conserve chlorine, volatilized tin chlorides could be treated to obtain hydrogen chloride, and the chlorides in treated residues could be transformed into hydrochloric acid by steam hydrolysis. Over 95 percent of the total tin in Bolivian slimes could be volatilized as chlorides, and this report describes comparable results in recovering tin from mine-run ores. Low-grade concentrates containing 10 to 25 percent of tin could be treated similarly to avoid substantial losses of the metal in slimes formed in producing high-grade concentrates. Part of the cost of producing a low-grade concentrate could be covered by the heat saved in treating less material containing a higher percentage of tin.
The relatively low boiling points of the tin chlorides (SnCl2, 623° C.; SnCl4 , 114° C.) have suggested volatilization as a means of separating tin from other materials. In 1917 Richards mixed pulverized tin ore with salt and a reducing agent and exposed the mass to a high temperature to volatilize tin as a chloride. Three years later, Collins recommended volatilizing tin as a chloride by roasting tin ore to remove sulfur and arsenic, reducing and cooling the roasted material in an inert atmosphere, contacting the mass with chlorine gas, and heating the mixture to bring about volatilization.
Rondelli obtained patents in 1923-24 for chloridizing tin ores at relatively high temperatures by contact with a fixed ratio of carbon monoxide and hydrogen chloride or carbonyl chloride. In 1926 Sulman and Picard described mixing oxidized tin-bearing ores and slimes with carbonaceous material and treating the mixture with hydrogen chloride under reducing conditions. Tin chloride was volatilized at 600° to 700° C. In 1934 Wood and Sulman modified this method by treating a mixture of tin ore and carbonaceous material with calcium chloride solution before volatilizing the tin chloride. Ashcroft obtained patents in 1928 and 1932 for chloridizing cassiterite with (1) zinc chloride or ferrous chloride in presence of zinc or iron and (2) ammonium chloride and a metal, such as iron. Tin chloride was volatilized at temperatures above 600° C.
Hughes patented a process in 1934 for treating various ores, including tin ores, with hydrogen chloride or chlorine, adding ferrous oxide, and again chlorinating the mixture. In 1937 Chizhikov and Balikhina suggested recovering tin from low-grade tin concentrates by chlorination to obtain a purer metal than that obtained by pyrometallurgy without using a refining step. They recommended contacting chlorine and carbon monoxide with charges of tin ore mixed with carbon and ferric oxide and heating the mixture at 400° to 600° C. Tin chloride was volatilized at 800° C., and recoveries of 98 percent of the total tin were claimed. In 1941 Frents utilized the same general procedure, using calcium chloride, magnesium chloride, or carnellite for the high-temperature chlorination.
In 1944 Muskat patented a procedure for passing chlorine containing about 3 percent oxygen over tin ore mixed with carbon. Tin chloride was volatilized at 800° C. In 1949 Malan described the volatilization of tin chloride from complex Bolivian ores by the Ashcroft-Elmore process. In this procedure a solution of ferric chloride was added gradually to a mixture of tin ore and powdered coal in a rotary drier. The product was heated to 850° C. to volatilize tin chloride, and the tin was recovered by electrolysis.
The principal tin-bearing mineral in tin ores, cassiterite (SnO2), is resistant to chemical attack. Thermodynamic calculations indicate that the reaction of cassiterite with either chlorine or hydrogen chloride at temperatures below 900° C. is endothermic. On the other hand, the reaction of stannous oxide (SnO) with these reagents below 900° C. shows a definite release of energy. Therefore, reduction of cassiterite to stannous oxide is desirable in forming tin chlorides at moderate temperatures.
In the experimental work described in this report, hydrogen is used as a reducing agent because it readily penetrates the crystal lattice of cassiterite. As both hydrogen and hydrogen chloride are gaseous under the operating conditions, the ratio of one to the other is adjustable and their recirculation is possible.
The principal reaction is given by the equation:
SnO2 + Hg + 2HCl → SnCl2 + 2H2O.
The relatively low temperature of the process results partly from a displacement of the equilibrium that tends to develop between solid and gaseous stannous chloride in the reaction zone. This displacement is controlled by the velocity of the gaseous mixture.
The following petrographic descriptions were obtained for the different tin ore concentrates and mine-run ores:
Banco Concentrate
This material was essentially cassiterite (SnO2), pyrite (FeS2), and quartz (SiO2), with earthy hematite (Fe2O3), iron-stained clay, and jarosite (K2Fe6 (OH)12(SO4)4). Traces of garnet (Al2O3 · 3FeO · 3SiO2), goethite (Fe2O3·H2O), zircon (ZrSiO4), and covellite (CuS) were present.
Much of the cassiterite was free of quartz and pyrite at minus 35-mesh; however, some locking of cassiterite grains with other minerals occurred at minus 200-mesh. Approximately 42 percent of the concentrate was coarser than 100-mesh, and about 28 percent was minus 325-mesh.
Colquiri Concentrate
This concentrate was composed mainly of siderite (FeCO3), cassiterite, and fine-grained sericitic mica, quartz, and fluorite (CaF2). It also contained small amounts of pyrite and pyrrhotite (Fe5S6 to Fe16S17) and traces of tourmaline, a complex silicate.
Tasne Ore
Mineralogical examination of this ore indicated essentially sericitic material, quartz, pyrite, and siderite. Trace amounts of hematite and magnetite (Fe3O4) were present. Most of the cassiterite was free at plus 100-mesh. Approximately 30 percent of the ore was finer than 325-mesh.
Bolivian Tin-Tungsten Ore
This ore consisted of cassiterite, pyrite, chalcopyrite (CuFeS2), siderite, hematite, and wolframite ((FeMn)WO4) with traces of scheelite (CaWO4). The gangue was essentially quartz and mica-tourmaline schist. Approximately 70 percent of the ore sample was plus 28-mesh, and less than 1 percent was minus 200-mesh. Most of the cassiterite and wolframite were free of gangue at minus 14-mesh.
Cerro de Potosi Ore
This ore was composed of pyrite, quartz, and sericitic mica, with small amounts of cassiterite and topaz, a complex aluminum silicate. Much of the cassiterite was free at minus 100-mesh. Table 1 gives chemical analyses of these concentrates and mine-run ores.
Experimental Procedures
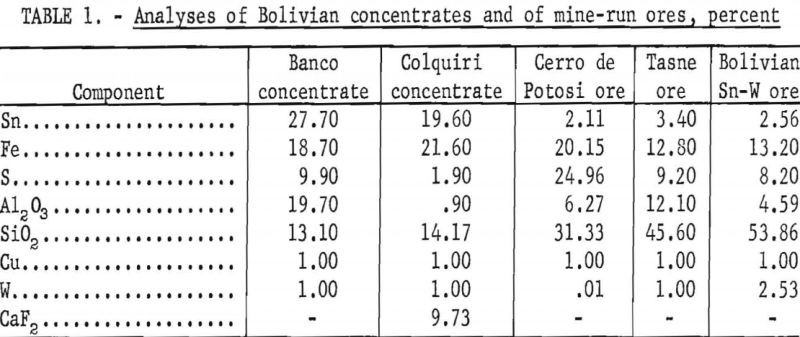
Charges of tin-bearing materials were treated with gaseous mixtures of hydrogen and hydrogen chloride, using: (1) a quartz tube inserted in a tube furnace and (2) an oscillating kiln equipped with a condensing train.
Procedure 1
A 50-gram charge of minus 35-mesh material was distributed evenly within a 12-inch reaction zone of a quartz tube having a length of 36 inches and a bore of 2 inches. The tube and charge were heated in an electric resistance-type furnace 27 inches long. Gases were metered, mixed, and passed through the reaction zone to an external water-cooled trap and a bubble bottle containing concentrated hydrochloric acid. Various rates of flow were used for the mixed gases. Charges were treated at temperatures ranging from 500° to 600° C. for different time intervals. About 15 minutes was required to bring the furnace and charge to the selected temperature. Tin chlorides were volatilized, swept from the reaction zone by flowing gases, and condensed in the exit train. Treated residues were weighed and analyzed for tin by a standard procedure.

The amount of tin volatilized was determined by measuring the difference in the amount of tin in the feed and that remaining in the residue.
Procedure 2
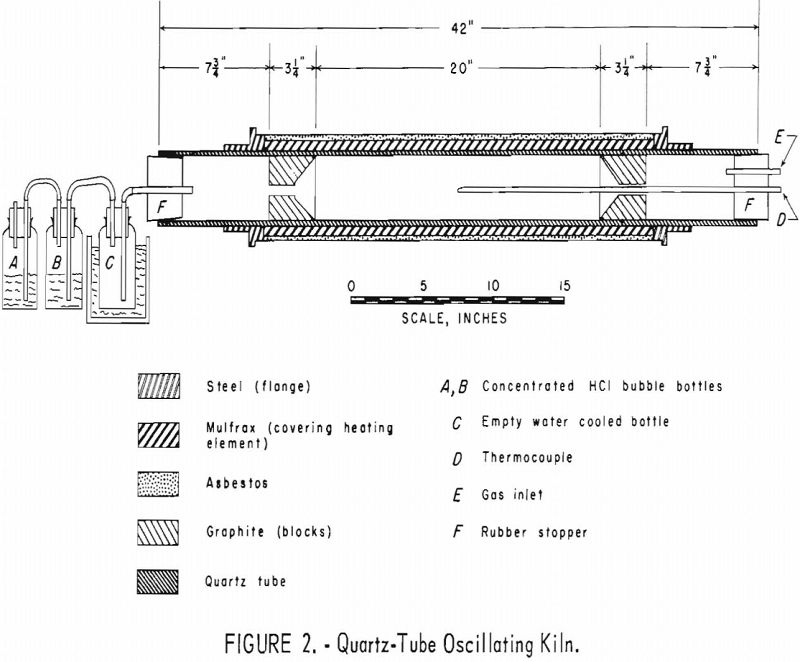
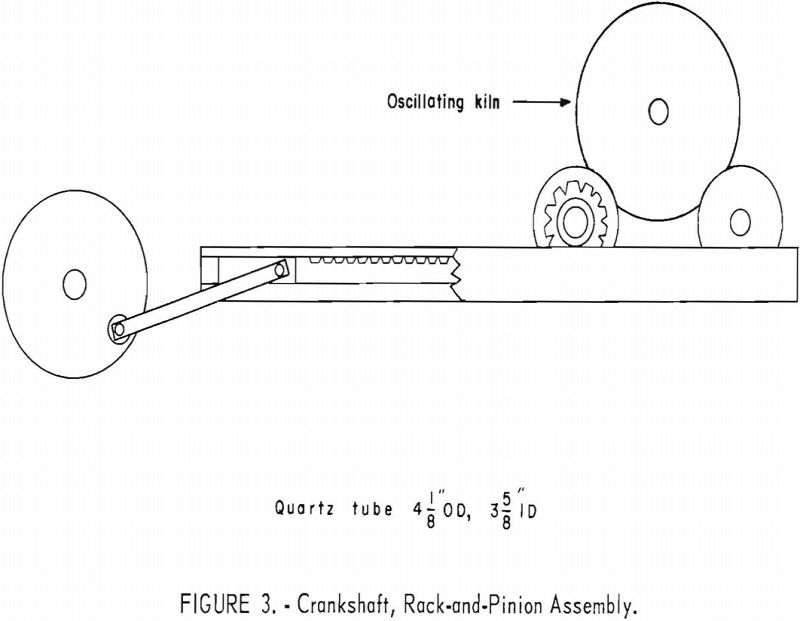
Experiments were conducted with larger charges of tin-bearing concentrates and mine-run ores in an electrically heated oscillating kiln (fig. 1). The kiln comprised a quartz tube surrounded by a heating coil embedded in a refractory (fig. 2). Concave graphite blocks were fitted inside the tube to retain the charge in the reaction zone. Holes in the block at the entry end of the kiln permitted flow of gases and provided an opening for a thermocouple. A hole in the center of the block at the exit end allowed the escape of tin chlorides and excess hydrogen chloride. A crankshaft rack- and-pinion assembly (fig. 3) provided 3-¼ complete oscillations per minute of the kiln through an arc of 180°. Gases were metered and passed through the kiln to a series of traps and to a bubbler containing concentrated hydrochloric acid. Tin chlorides were volatilized, swept from the reaction zone by flowing gases, and collected in the exit train. Most of the tin chlorides were trapped in the water-cooled bottle.
After each experimental run, the graphite blocks were washed with hydrochloric acid. Tin was not detected in the washings, however, until a substantial number of runs had been completed. The blocks were then replaced by new ones. Treated residues remaining in the kiln and the volatilized products were analyzed chemically by a standard method. Material balances were satisfactory.
Experimental Results
Procedure 1
Experiments were run on each concentrate and mine-run ore to study the effects of different conditions of treatment. Variations in the required temperatures for the reaction zone depended on such factors as the size of cassiterite grains and their distribution in the gangue. The results for each material are summarized in the following paragraphs.
Banco Tin Concentrate
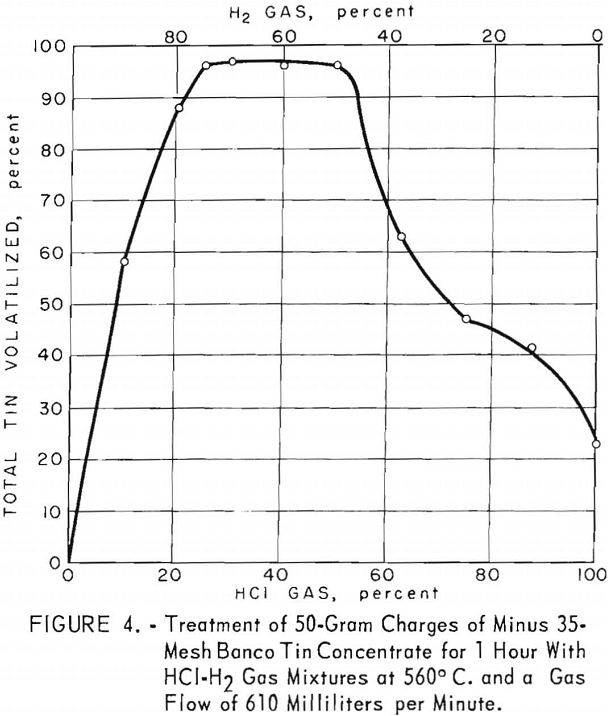
The composition of hydrogen and hydrogen chloride gas mixtures was plotted against the total tin volatilized in chlorides at 560° C. using a gas flow of 610 milliliters per minute for 1 hour (fig. 4). Optimum gas mixtures for this concentrate contained 25 to 30 percent hydrogen chloride. Within this percentage range, approximately 96 percent of the total tin was volatilized.
Colquiri Tin Concentrate
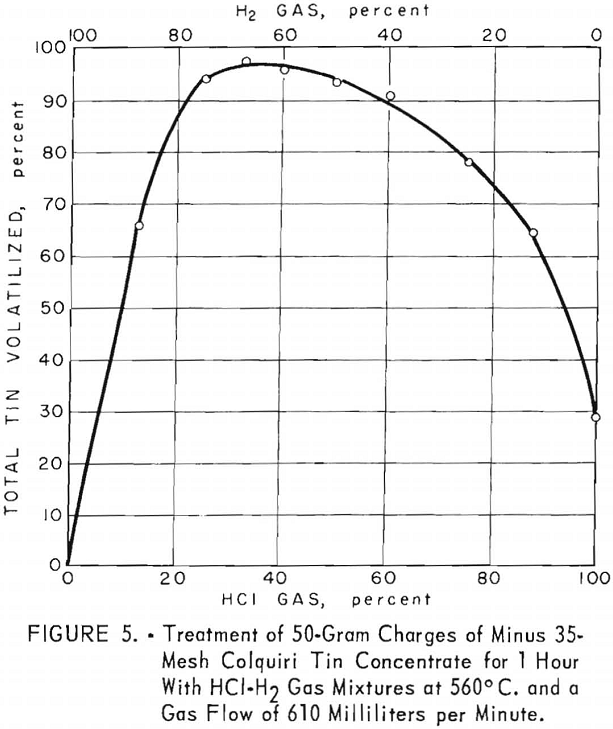
Optimum gas mixtures for treating this concentrate contained 30 to 40 percent hydrogen chloride (fig. 5). Results were somewhat similar to those obtained for Banco concentrate, except that yields of tin decreased less rapidly after passage through the most effective range of gas mixtures. About 96 percent of the total tin was volatilized as chlorides at 560° C., using a gas flow of 610 milliliters per minute for 1 hour.
Tasne Mine-Run Ore
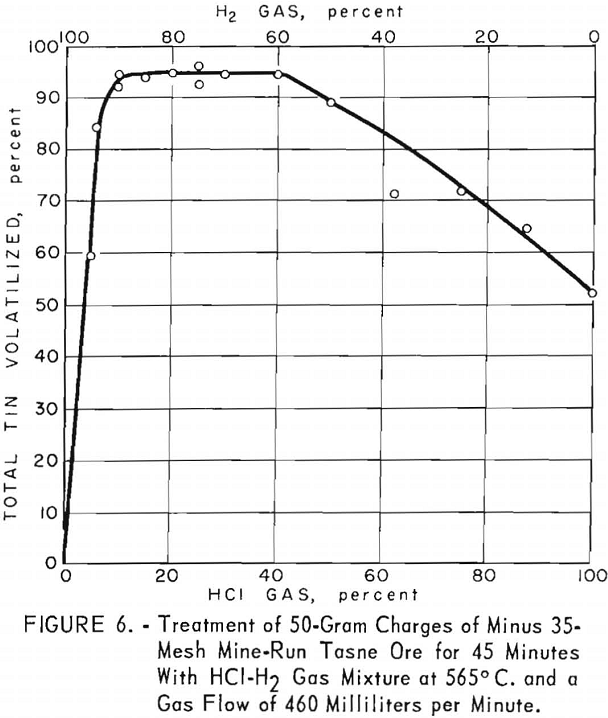
Approximately 95 percent of the total tin in this ore was volatilized at 565° C. Gas mixtures containing 10 to 40 percent hydrogen chloride (fig. 6) were optimum when a flow of 460 milliliters per minute was used for 45 minutes. As the tin content in this ore was substantially lower than that in the Banco concentrate, a shorter time of treatment was required with gas mixtures having less hydrogen chloride and a slower rate of flow. When the temperature of the reaction zone was lowered to 540° C., the yield of tin decreased to 84 percent.
Bolivian Tin-Tungsten Mine-Run Ore
As shown in figure 7, the optimum proportion of hydrogen chloride in gas mixtures was very broad (from 10 percent upward). Yields of 95 percent of the total tin were obtained at 520° C., using a gas flow of 460 milliliters per minute for 45 minutes. The temperature of 520° C. was effective, compared with 565° C. for Tasne ore treated under otherwise similar conditions.
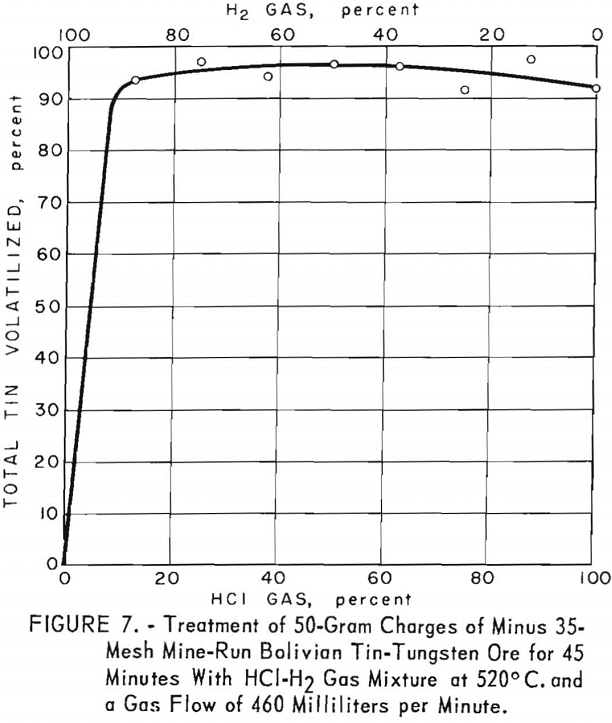
Although the ore contained 2 to 3 percent tungsten, practically all of the tungsten remained in the treated residues.
Cerro de Potosi Mine-Run Ore
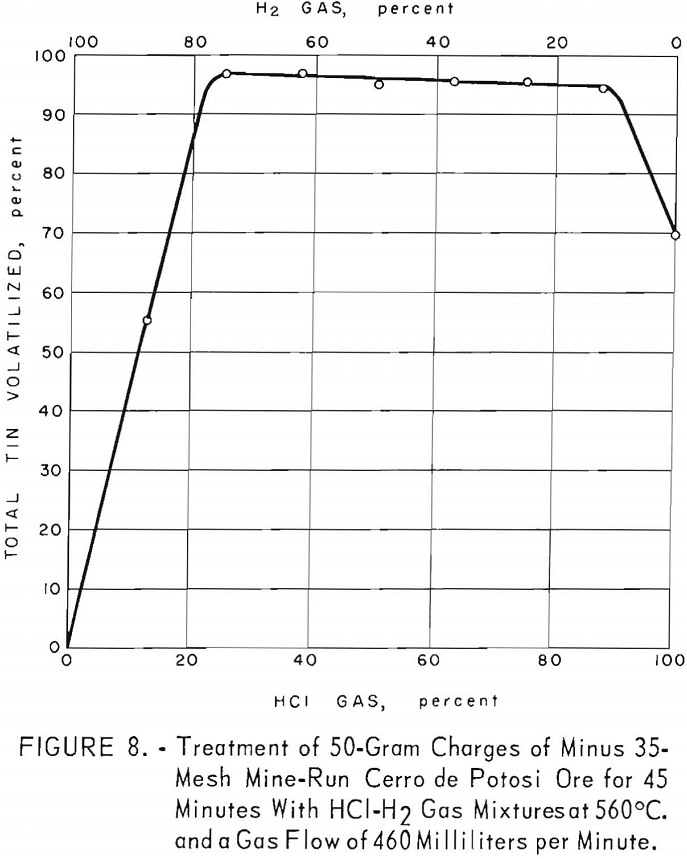
This ore contained much more iron and sulfur and less silica than either of the other two mine-run ores. (See table 1.) More than 95 percent of the total tin was volatilized as chlorides at 560° C. (about the temperature used to obtain a maximum yield of tin from Tasne ore). The optimum range of hydrogen chloride in the gas mixtures was 25 to 88 percent (fig. 8), using a flow of 460 milliliters per minute for 45 minutes. This range was narrower in percentage of hydrogen chloride than that used for Bolivian tin-tungsten ore.
A few experiments were run with the different tin-bearing materials using gas mixtures in which 3 to 9 percent of the hydrogen was replaced by hydrogen sulfide. Under otherwise similar operating conditions, yields of tin were increased 2 to 3 percent over yields with Colquiri tin concentrate, Tasne mine-run ore, and Cerro de Potosi mine-run ore. Tin recoveries from the Banco tin concentrate and Bolivian tin-tungsten ore were not improved by including hydrogen sulfide in the gas mixtures.
Procedure 2
Preliminary tests were made with the oscillating kiln using kilogram charges to establish general operating conditions. Comparing the results by charge
weight, it was found that the same or slightly higher rate of gas flow, a considerably shorter time of treatment, and a higher content of hydrogen chloride in the gas mixtures were needed in relation to optimum conditions for procedure 1. Temperature requirements were approximately the same. Charges were composed of minus 35-mesh concentrate or mine-run ore.
Banco Tin Concentrate
Because of the high tin content of this concentrate (table 1), kilogram charges produced more tin chlorides than the exit assembly could collect without clogging. To avoid this operational difficulty, charges were reduced to 500 grams.
Table 2 summarizes data for treating Banco concentrate. Optimum conditions were attained in tests 1 and 2, in which 96 to 97 percent of the total tin was volatilized as chlorides. When the time of treatment was reduced from 5 to 4 hours and other conditions were the same (tests 3 to 6), yields of tin were much lower. With a smaller percentage of hydrogen chloride in the gas mixtures (tests 7 and 8) than in tests 1 and 2, tin recoveries were less. When the rate of flow of gases was reduced from 1.2 to 1 liter per minute (tests 9 and 10) and other conditions were optimum, a smaller quantity of tin chlorides was volatilized from the charge. Yields of tin were affected more by reducing time of treatment and rates of gas flow than by lowering the percentage of hydrogen chloride in the gas mixtures.
Colquiri Tin Concentrate
In contrast with other tin-bearing materials listed in table 1, this concentrate contained a sizable amount of calcium fluoride. The presence of this compound led to serious corrosion of the quartz tube in the kiln; consequently, the number of experiments was limited. Data are summarized in table 3.
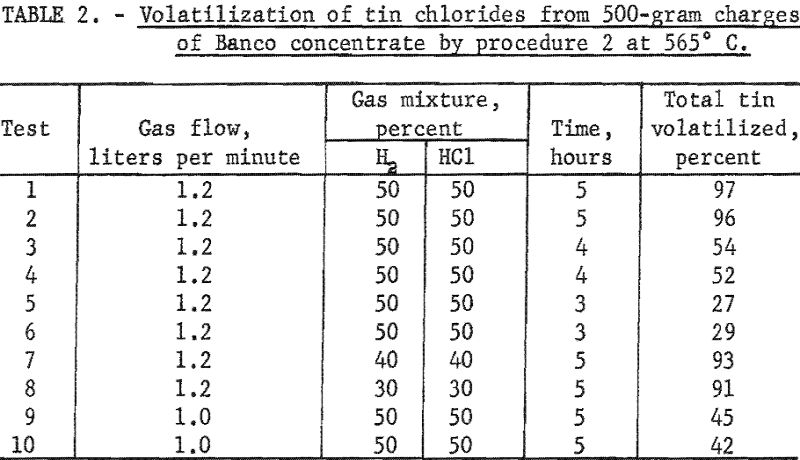
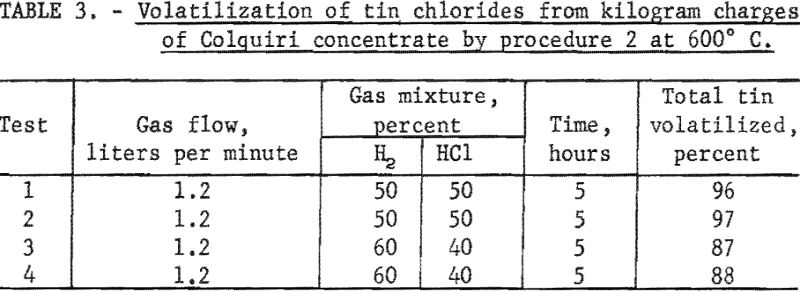
A 5-hour treatment with a 50 : 50 mixture of hydrogen and hydrogen chloride at 600° C. (table 3, tests 1 and 2) gave high yields of tin chlorides. When the percentage of hydrogen chloride in the gas mixture was reduced from 50 to 40 percent and other conditions were kept constant (table 3, tests 3 and 4), less tin was volatilized as chlorides.
The data obtained for this concentrate by procedure 1 suggested that results might be equally satisfactory at lower reaction temperatures. Owing to corrosion difficulties, this possibility was not verified. The addition of silica to charges of the concentrate did not provide adequate protection for the quartz tube in the kiln.
Tasne Mine-Run Ore
This ore had the highest tin content (table 1) of the mine-run ores used in experimental work. Optimum conditions for treating the Tasne ore were attained in tests 2 and 9. (See table 4.) Because the tin content of this ore was substantially lower than that of the Banco concentrate, the time of treatment was considerably less than that for the Banco material (fig. 9, table 4, tests 2 to 4). The rate of gas flow through the reaction zone also affected the yields of tin (table 4 tests 3, 4, and 6).
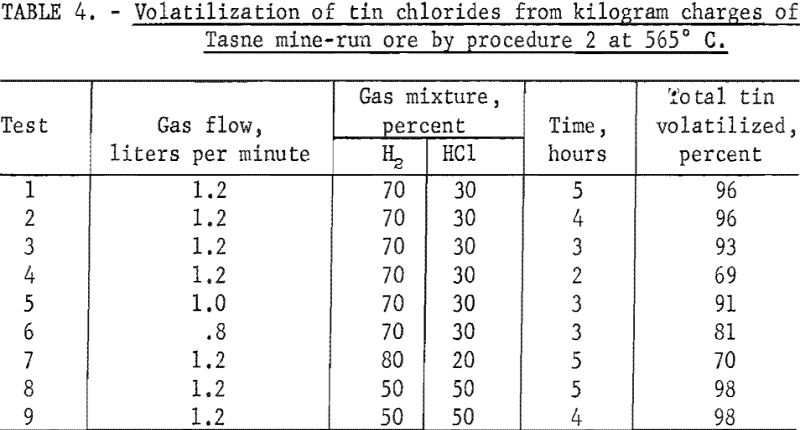
When the hydrogen chloride content of the gas mixture (table 4, test 1) was reduced (table 4, test 7), less tin was recovered. A higher percentage of hydrogen chloride in the gas mixture (table 4, tests 8 and 9) increased volatilization of the tin. over that in tests 1. and 2, table 4.
Bolivian Tin-Tungsten Mine-Run Ore
As pointed out under procedure 1, this ore differed from the other mine-run ores in that it contained 2 to 3 percent of tungsten. In experimental runs, nearly all of this element remained in the treated residues. Optimum conditions for volatilizing tin chlorides from the ore are summarized in table 5, tests 1 and 2. A reaction zone temperature of 520° C. was adequate, compared with 565° C. for Tasne ore. Reducing the time of treatment to less than 4 hours (table 5, tests 3 and 4) and keeping other operating conditions the same caused decreases in the quantity of tin volatilized (fig. 9). Similar tests in which the reaction time was increased to 5 hours (table 5, test 5) did not improve the yield of tin over that in test 2, table 5.
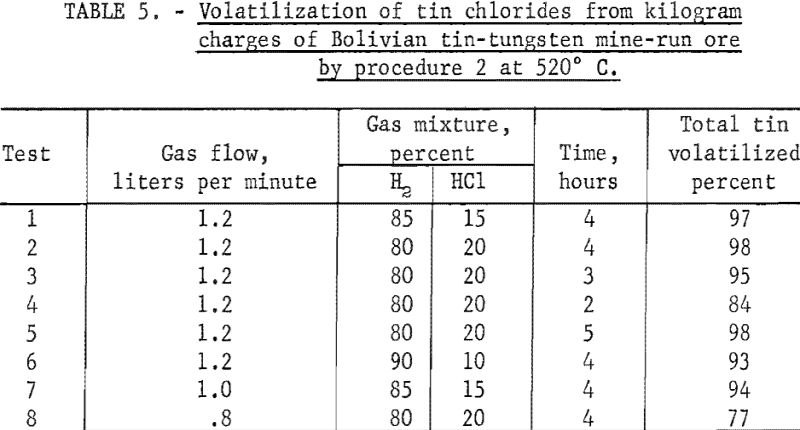
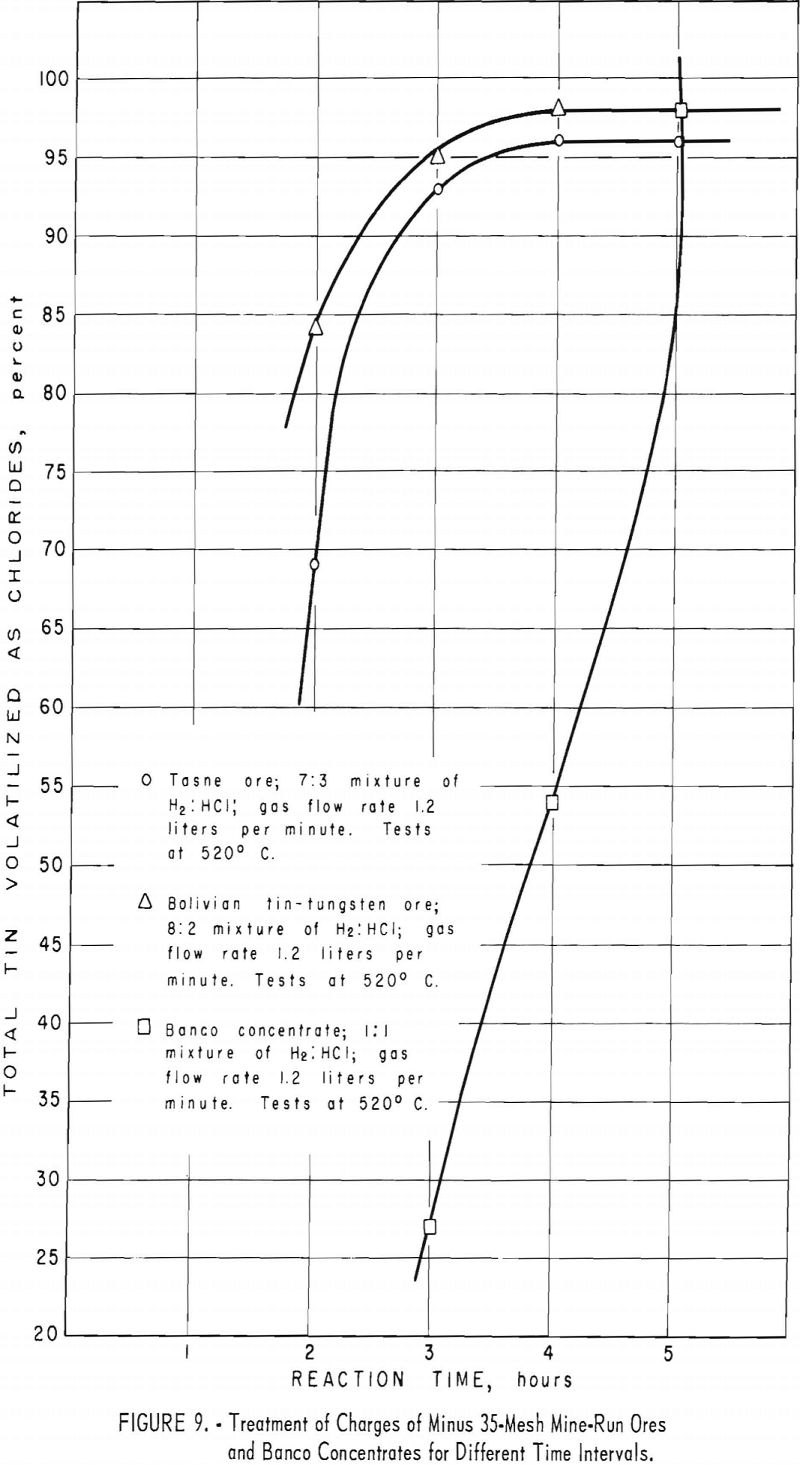
When the ratio of hydrogen to hydrogen chloride was changed from 85 : 15 (table 5, test 1) to 80 : 20 (table 5, test 2), the recovery of tin increased slightly. Further reduction in the ratio of hydrogen to hydrogen chloride to 90 : 10 (table 5, test 6) gave lower yields of tin.
When the rate of flow of the gas mixture was reduced from 1.2 liters per minute (table 5, test 1) to 1 liter per minute (table 5, test 7) and other operating conditions were the same, the volatilization of tin declined about 3 percent. Likewise, a reduction in flow rate to 0.8 liter per minute (table 5, test 8) caused a decrease in tin recovery.
Cerro de Potosi Mine-Run Ore
This ore was the only one of the five tin-bearing materials used for experimental testing that required more than 50 percent of hydrogen chloride in the gas mixtures to obtain high percentage volatilization of the tin as chlorides. As shown in table 1, this ore contained the highest percentage of sulfur. Most of the sulfur was in the form of pyrite, causing the ore to have a higher percentage of iron than the other two mine-run ores. At the reaction-zone temperature, the pyrite was converted to ferrous sulfide, which reacted with hydrogen chloride to form ferrous chloride and hydrogen sulfide.
The highest recovery of tin from Cerro de Potosi ore was obtained under conditions used for test 1, table 6. When percentages of hydrogen chloride in the gaseous mixtures were below 85 percent (table 6, tests 2 to 5) and other operating conditions were the same as those for test 1, table 5, yields of tin were correspondingly reduced.
When the rate of flow of the gas mixture was reduced from 1.2 liters per minute (table 6, test 3) to 0.8 liter per minute (table 6, test 6), tin recovery declined substantially.
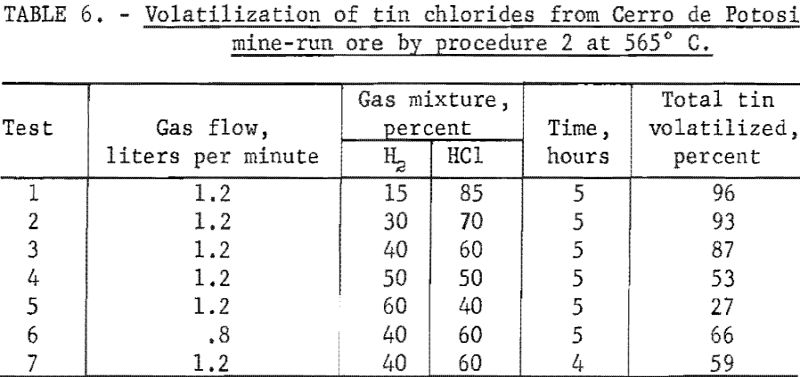
Reducing the time of treatment from 5 hours (table 6, test 3) to 4 hours (Table 6, test 7) and keeping other conditions the same caused a marked decrease in volatilization of the tin as chlorides.
The higher tin content of Tasne ore (table 1) caused a rather abrupt drop in the percentage of tin volatilized as the percentage of hydrogen chloride in the gas mixtures was decreased (table 4, tests 1 and 7). This decrease was not so sharp for Bolivian tin-tungsten ore (table 5, tests 1 and 2) or Cerro de Potosi ore (table 6, tests 1 and 2).
