Table of Contents
This work defines and systematizes the physical factors of the problem of leaching and demonstrates for the first tine the utility of macropore volume, micropore volume., specific surface, pore size distribution and permeability as parameters of leaching based on a study of the recovery of uranium from the Top Black member of Chattanooga Shale.
A set of factors which govern the leaching of ores was defined and organized into a logical system based on the elements of leaching; namely, accessibility, physico-chemical interaction and transport. From this array a set of categories was chosen which permitted a regrouping as follows:
- Structural factors
- Fluid factors
- Factors common to fluid and structure
- Operational factors
- Chemical factors
Elements of Leaching
Accessibility is a vital element because interaction between the desired constituents and the leaching fluid cannot take place in the absence of contact.
The necessary sub-elements to establish contact between constituents and fluid are exposure and penetrability. Exposure is the opening up of the desired constituents and penetrability is the ability of the leaching fluid to penetrate to sites where desired constituents are exposed.
Exposure depends on volume, shape and location distributions of the values of a given mixture. Given two mixtures differing only in the volumes of their values, the one having the larger mil be opened to possible penetration by a given fluid at a larger particle size. If volumes and shapes are the same, the mixture whose values are located along internal openings or planes of cleavage will be exposed at a larger size than one in which they are imbedded in a homogeneous structure.
Capillary pressure is the force per unit area across the curved interface between two immiscible fluids, at least one of which is a liquid. For a cylindrical pore of diameter D, Washburn relates this pressure to pore diameter, liquid surface tension and liquid-solid contact angle. Thus, we have
Pc = 4 r cos θ/D
where, Pc is capillary pressure across the meniscus, r is liquid surface tension and θ is liquid-solid contact angle through the liquid phase. If the capillary pressure is negative, such as is the case when 0 is greater than 90°, positive external pressure must be applied to get the liquid to enter the pore opening. This pressure must be at least greater than the value given by the calculation of Pc.
Viscosity is generally not an important factor because rates of entry into pores are usually low and consequently shear resistance offered practically negligible. However, if openings are exceptionally small, considerable pressure might have to be exerted to cause penetration by highly viscous fluids.
Establishment of contact between values and lixiviant does not alone insure successful leaching because it is necessary to convert the desirable constituents from a fixed to a mobile condition. This may take place by direct solution or volatilization of constituents or in consequence of a reaction with one or more components of the fluid which yields a soluble or volatile reaction product which goes.into solution simultaneously or subsequent to formation.
Accessibility and physico-chemical interaction by themselves do not assure successful leaching without transport of products away and reactants to the zone of interaction. Diffusion and convection are the two fundamental flow processes involved.
For porous materials concentration gradient at the exit of a pore under steady state flow conditions may be approximated by dividing the solubility of the desired constituent in terms of concentration minus its concentration in the main body of the lixiviant by the length of path from its origin in the pore to the exit.
Recovery of Uranium from the Top Black Member of Chattanooga Shale by Leaching
The Top Black consists essentially of a clay-organic complex matrix in which are imbedded various inorganic granular materials arranged in a compact series of fine beds. The uranium content is less than 0.009% and submicroscopic so that standard optical techniques cannot be used to observe its association with other shale minerals. This makes the analysis of exposure and penetrability more difficult than if direct observation techniques could be employed.
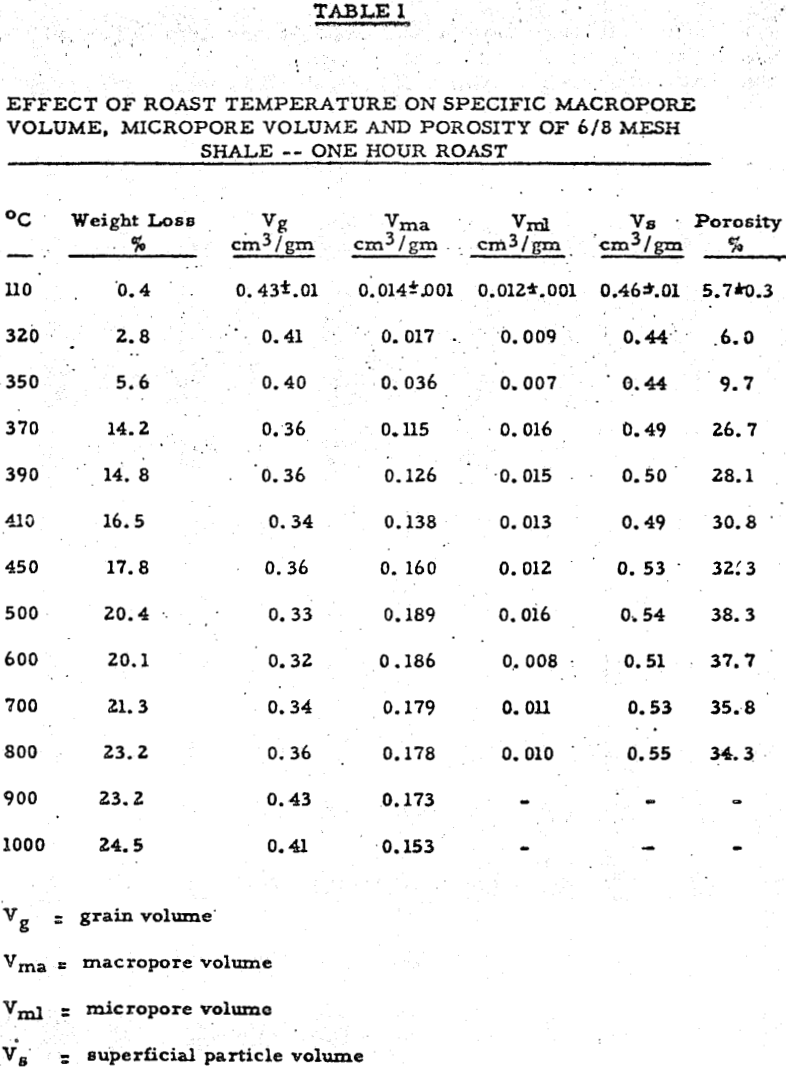
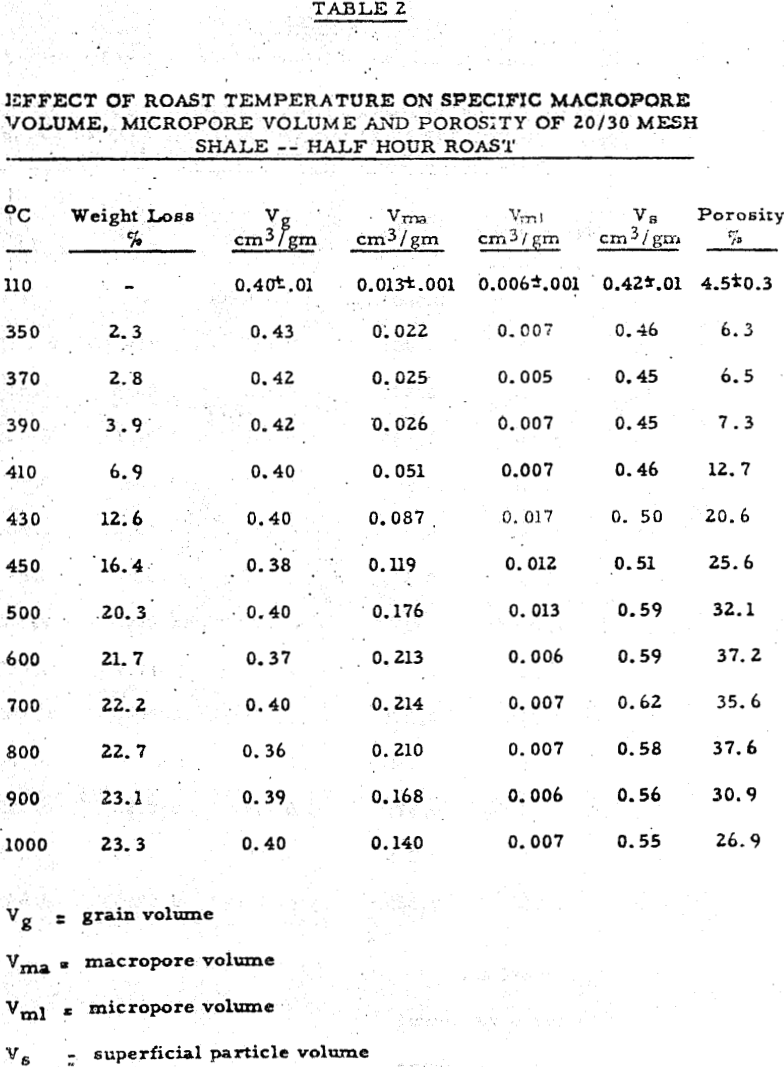
Sized shale samples were placed in a shallow fused silica dish to a depth of one to two layers and roasted for various periods of time at different temperatures in a type B-2 Lindberg muffle furnace vented to the atmosphere through a small opening. Weighings were made of each lot before and after thermal treatment and percent weight loss calculated. Samples from each lot were then chosen for physical measurement. Extraction data were obtained at room temperature by leaching five gram samples of roasted shale from each lot in 100 cm³ of 5% by weight of sulfuric acid for a fixed time using glass beakers and stirrers. The lixiviant used was a good choice for the following reasons: (1) Practically all known uranium compounds with the exception of complex silicates are sufficiently soluble in this solution to permit dissolution of all the non-silicate uranium present in the shale. (2) The inorganic components which make up the bulk of the shale such as pyrite, hematite, mica, quartz, chlorite, feldspar, and illite are not soluble to any significant degree in this lixiviantThus, it is ideal for probing uranium exposed prior to leaching.
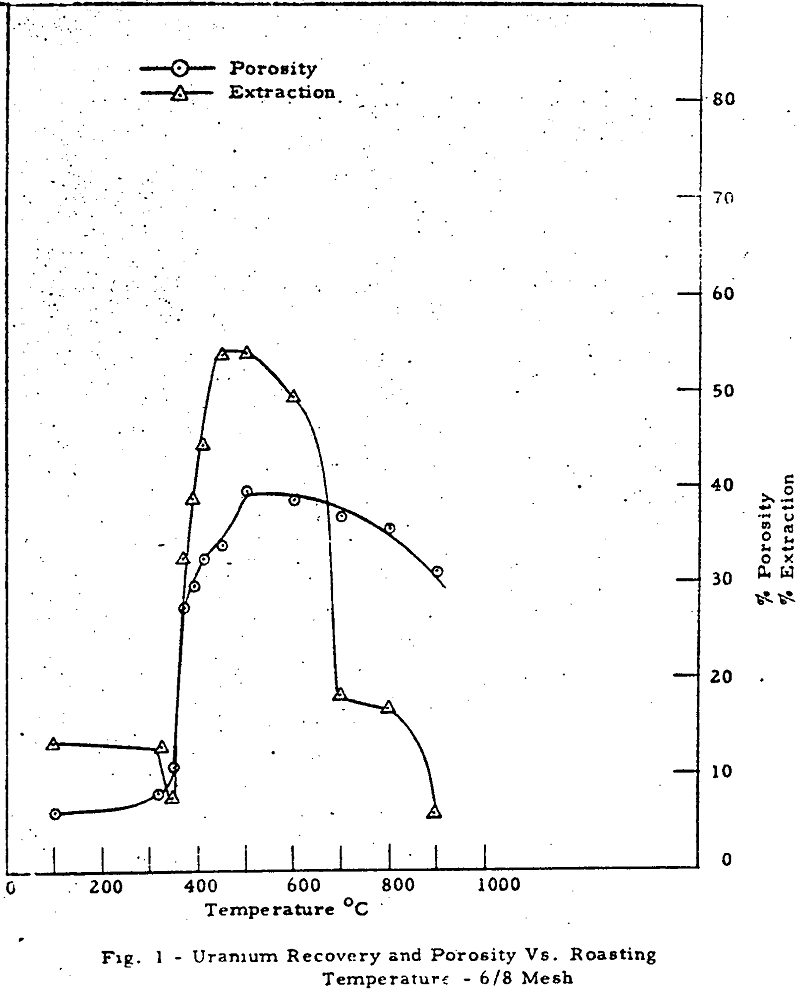
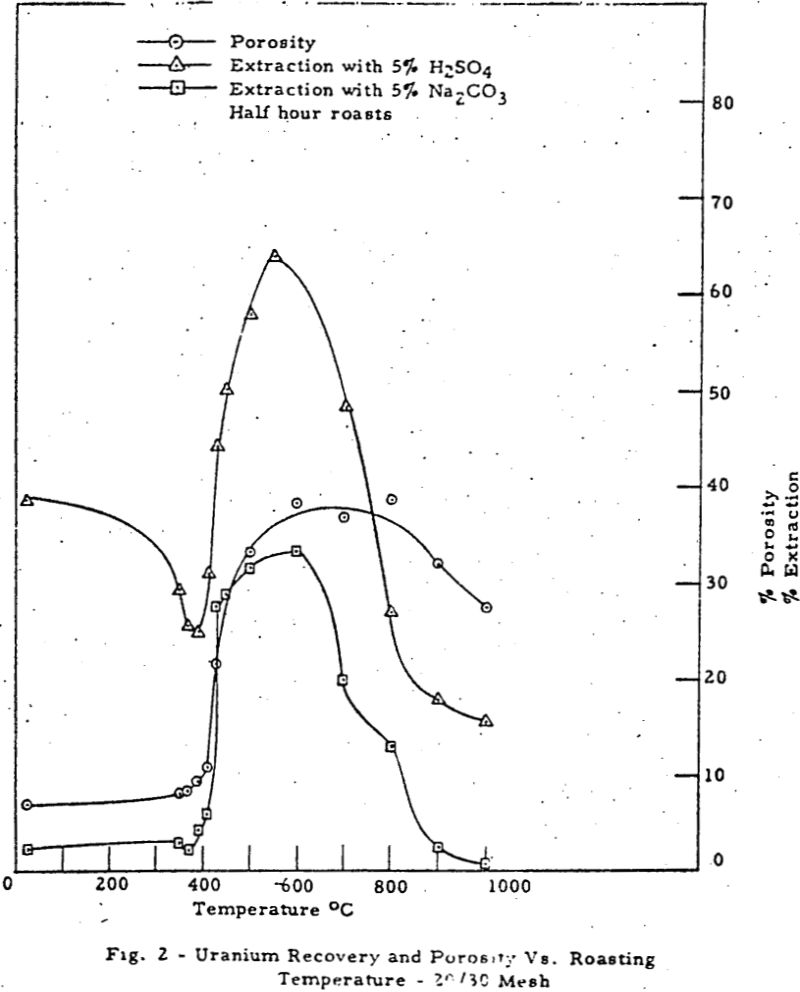
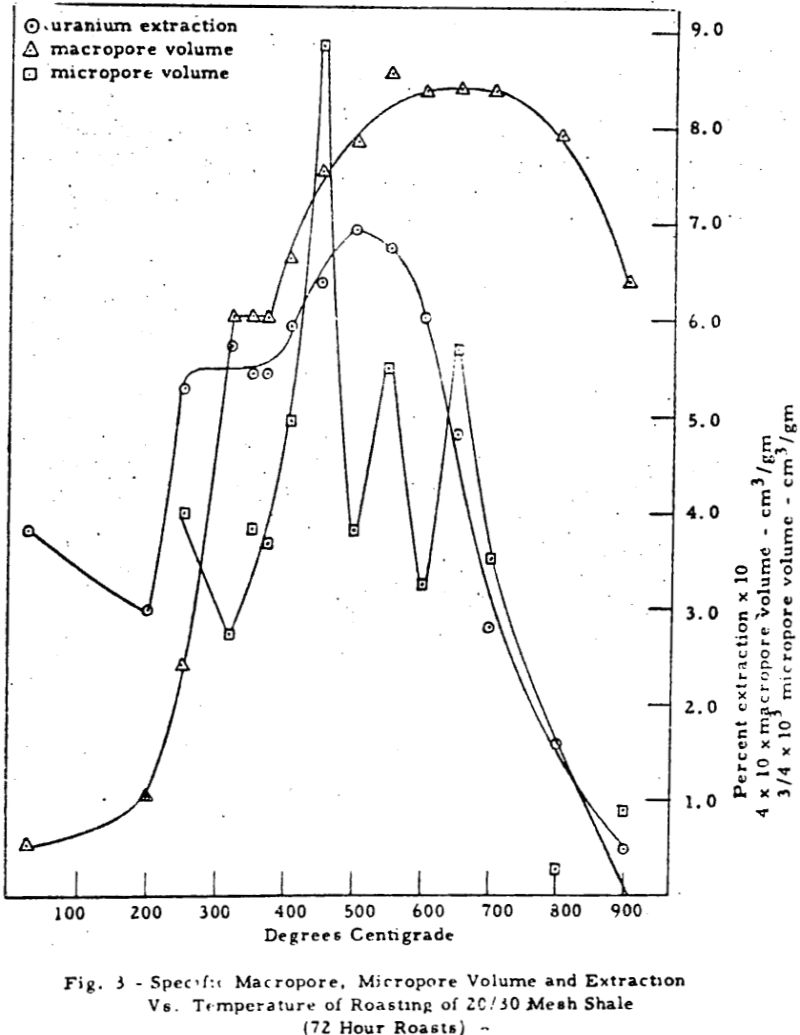
The results can be divided into three regions of interest. Region 1 (room temperature to about 370-390°C) shows a rise in porosity and decrease in extraction with temperature. Region 2 (370-390°C to 500-550°C) shows a parallel rise in both porosity and extraction. Region 3 (above 550-600°C) shows a mild decrease in porosity and a drastic drop in extraction with temperature.
The macropore volume curve is characterized by two flat regions. The first one commencing at 320°C coincides with the disappearance of most of the carbon and the second at 500°C with the disappearance of most of the sulfur,, Hence, macropore volume develops primarily as a result of carbon and sulfur removal.
Raw shale at 20/30 mesh was leached in beakers at room temperature for two hours with various concentrations of sulfuric acid. Macropore volumes of the residues were measured and compared with corresponding uranium extractions.
Macropore volume remains fairly constant for acid strengths ranging from 15% to 90% at about 0.014-0.016 cm³/gm and recovery fluctuates within limits about an average value of 35%. At an acid strength of 96% by weight the macropore volume jumps to 0.067 cm³/gm. and recovery to 68%. Since particle size remained unaltered for all acid concentrations, this suggests that for a given chemistry extraction is paralleled by macropore volume.
The problem of accessibility has been studied for roasted and raw shales. (1) It has been observed that for a given chemistry macropore volume parallels extraction of uranium from shale roasted in the 300°-500°C range for sufficient time and conversely, for a given macropore volume extraction is determined by chemistry. (2) It has been observed that for a given chemistry extraction of uranium from shale roasted above 650°C for sufficient time is paralleled by micropore volume or specific surface and conversely, for a given micropore volume or specific surface extraction is determined by chemistry. (3) It has been observed that for a given chemistry, the uranium extracted from raw shale is paralleled by creation of macropore volume. (4) Since macropore volume, micropore volume and specific surface are factors of exposure and the latter is a sub-element of accessibility, one interpretation placed on the parallelisms noted is that for a given chemistry recovery, of uranium from the Top Black may be determined by accessibility and conversely, for a given accessibility it is determined by chemistry.