In portland cement manufacturing, the raw materials are changed to clinker in the burning operation. Generally the rotary kiln is used for this purpose and involves many processes including pelletizing and nodulizing. This transformation is called clinkering and is analyzed as now generally practiced in cement making from the raw material to the resulting clinker.
Cement manufacturing may be divided into two general kinds of processing: (a) crushing and grinding, and (b) turning, this is accomplished by means of a variety of operations, including excavation, drilling, blasting, hauling, conveying and pumping. Throughout the entire procedure of transforming non-metallic minerals to the finished cement, quality control plays an important role from the point of view of the consume as well as for optimum plant operation.
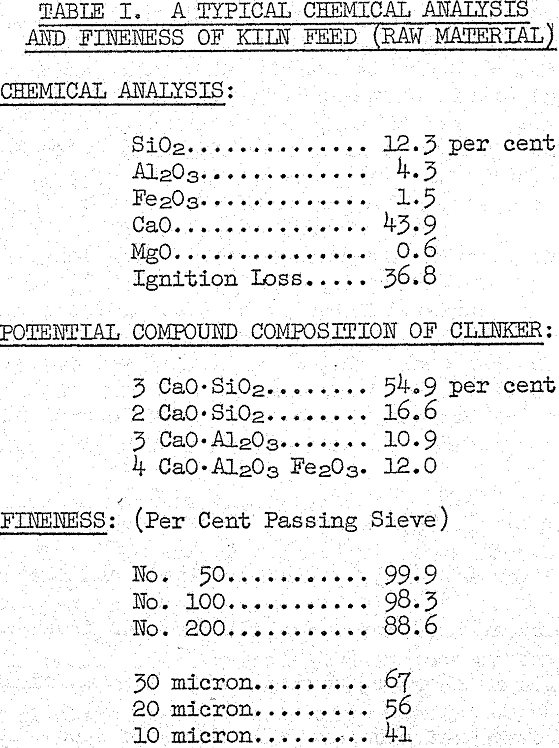
In chemical control the proportions of raw materials are modified to obtain a desired composition of the feed material for the burning operation. As part of this chemical mix design, allowance is made for ash from the fuel, which in coal firing, becomes part of the clinker. Coal ash contains the same oxides found in the raw materials. After the mixture of raw materials has been properly proportioned and ground to suitable fineness, the resulting raw mix becomes kiln feed.
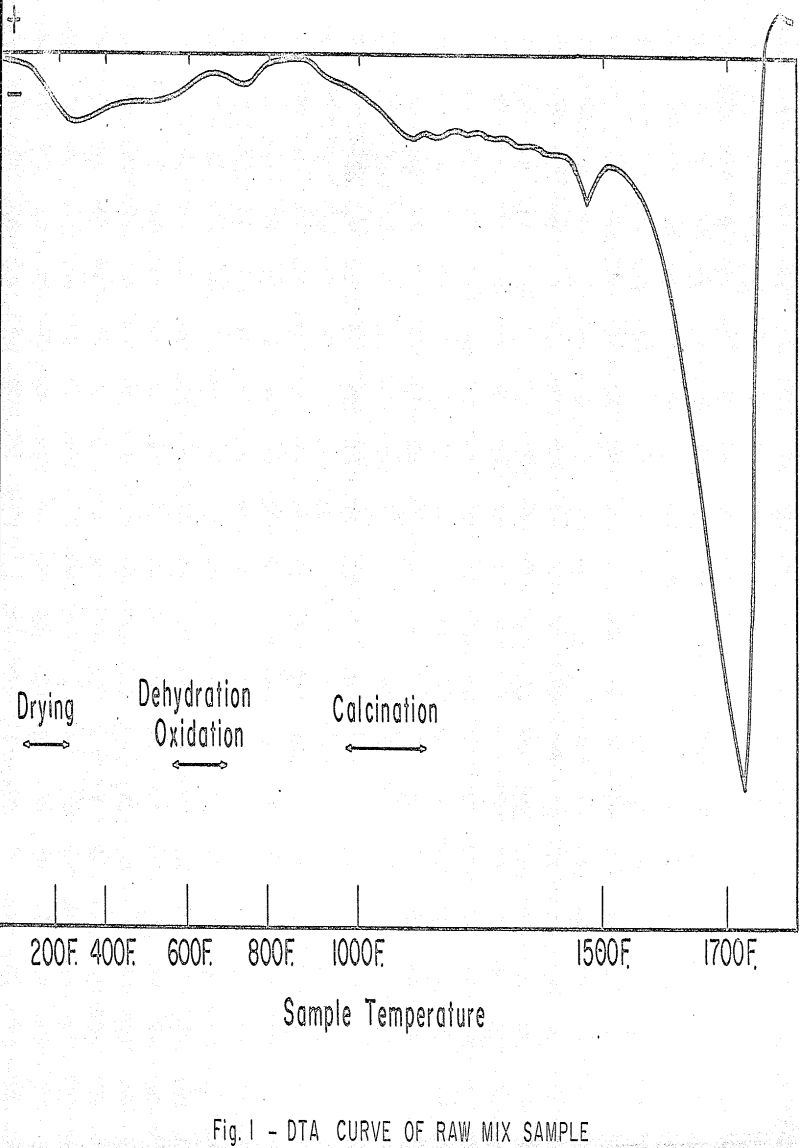
As the feed moves down through the kiln, the chemical process reactions begin to take place. This is considered to be:
- Drying or loss of surface moisture.
- Dehydration of non-metallic minerals such as clays or shales.
- Oxidation of certain carbonaceous substances which may be present, such as kerogens.
- Decomposition of MgCO3.
- Decomposition of CaCO3, which is the strongest reaction in the lower temperature range of the burning process.
- Formation of calcium silicates largely 2CaO·SiO2 as CaO becomes available.
- Formation of compounds of lime (CaO) and alumina (Al2O3) or iron (Fe2O3).
- Formation of calcium silicates from previously formed 2CaO·SiO2 and uncombined lime (CaO).
The amount of carbonate minerals present is sufficient to cause a large deflection of the curve, and thereby overshadow any lesser reactions taking place in this temperature range. The peak shown in the area near 800 C (1472 F) is found when a magnesian limestone is one of the components.
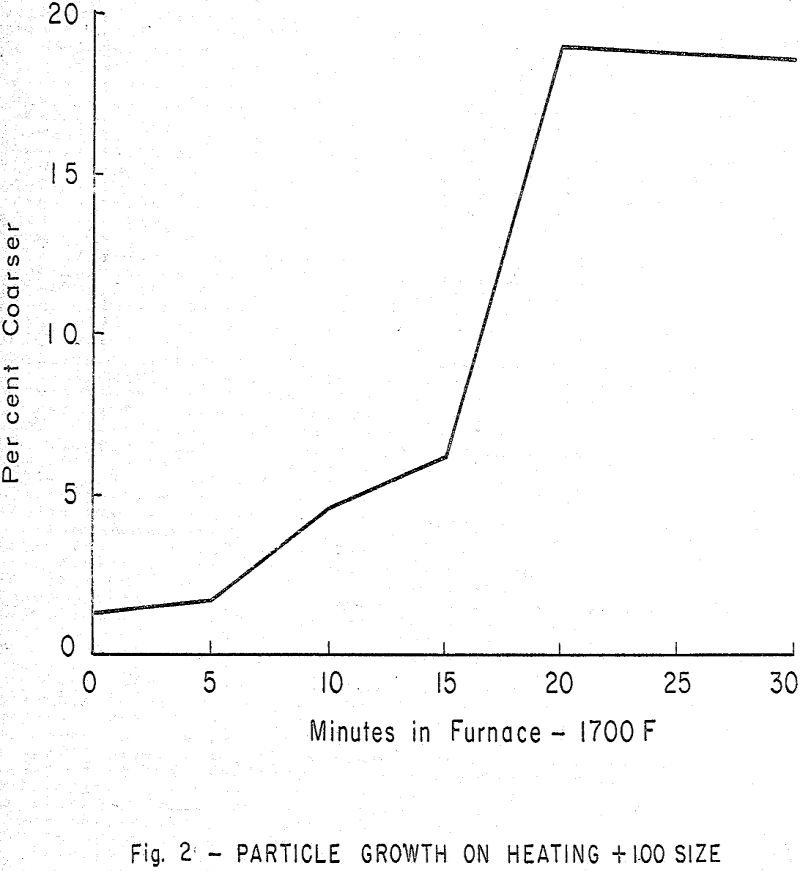
As the mix is heated to the range 1280 to 1350 C (2336-2462 F), liquid is formed by a portion of the constituents, finally reaching a typical value of 25 per cent. This would then introduce the nodulizing process into the kiln system. It may be shown that the liquid forms as an interstitial substance and serves as a basis for the final clinker pellets.
As the clinker in the kiln passes through the hot zone, its temperature is increasing, finally reaching a maximum at a point near the discharge end.