Table of Contents
Coal normally contains trace amounts of fluorine ranging up to about 0.02 percent. These small quantities of fluorine generally are volatilized when coal is burned in boiler furnaces. but they may be a transient factor in some types of corrosion and occasionally in deposit formation, and may contribute to atmospheric pollution.
A standard method is needed for the determination of fluorine in coal, and there is little published information on the occurrence of fluorine in U.S. coals. Churchill, Rowley, and Martin reported 0.0085 to 0.0295 percent fluorine in eight coals they analyzed, and Bradford found 0.0040 to 0.0132 percent in six western coals.
Crossley investigated the occurrence of fluorine in British coals and discussed its industrial significance. McGowan developed a spectrophotometric method of determining fluorine in coal using oxygen bomb washings. Kunstmann and others investigated fluorine and boron in South African coals, and Durie and Schafer studied fluorine and phosphorus in Australian coals.
- Calcium oxide can be used as a fixative agent for fluorine when ashing a sample of coal.
- A mixture of phosphoric and sulfuric acids is preferred for distillation of fluosilicic acid, but sulfuric acid also can be used alone.
- The SPADNS-zirconium colorimetric method is rapid, and can be applied readily for determination of fluorine in distillates.
- Tests of float- and sink-fractions showed that the fluorine was associated with mineral matter in the three coals examined.
- Fluorine and phosphorus contents could not be correlated, but coals having high phosphorus generally had the higher fluorine contents.
- Fluorine content of the commercial coals tested ranged from 0.001 to 0.019 percent of dry coal.
The methods of determining fluorine in these investigations were somewhat lengthy, so tests were carried out with the aim of shortening the procedures, and also of obtaining information on the range of fluorine content of commercial U.S. coals.
Methods investigated for determining trace amounts of fluorine in coal have three steps : (1) Ashing a coal sample with a fixative agent to retain the fluorine, and fusion of ash residue with sodium carbonate to decompose the minerals, (2) distillation of fluorine from an acidified solution of the melt, and (3) determination of the fluorine recovered in the distillate.
This report gives results of experiments with several modifications of the method, and includes results for fluorine content of 83 U.S. coals.
Spadns-Zirconium Method
A method of determining fluorine in the distillate obtained when testing coal was investigated. The bleaching action of fluorine on the color of zirconium-alizarin complex or thorium-alizarin complex has been used, and the method of Belcher, Leonard, and West as modified by Johnson and Leonard was applied in testing Australian coals. A disadvantage of these methods is that several reagents are required in testing an aliquot of the distillate, and the final test solution must stand 1 hour for color development. Titration methods using sodium alizarin sulfonate indicator also are available.
Several colorimetric methods for determination of fluorine in public water supplies have been developed; the SPADNS-zirconium method described by Bellack and Schouboe was selected for trial. Only one reagent solution is required, and it can be added directly to an aliquot of the distillate when testing coal. Bleaching action of any fluorine present on the colored solution is rapid and the absorbance reading can be made in a few minutes, after adding the reagent. Details of the method are given in the appendix.
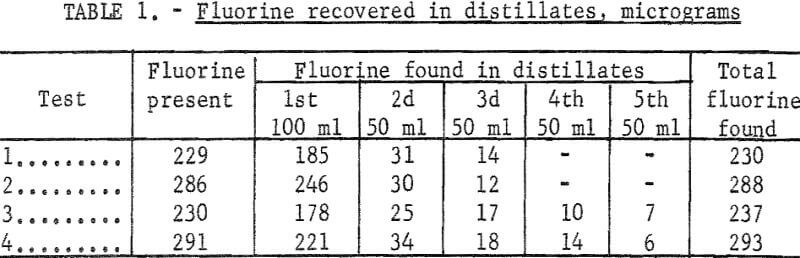
This method was used to determine the amount of fluorine recovered in the distillate when testing solutions containing about 200 to 300 micrograms of fluorine. Bureau of Standards opal glass sample No. 91 containing 5.72 percent fluorine was used to prepare the test solutions. Samples weighing from 0.05 to 0.10 gram were fused with sodium carbonate, and the melt was leached with water and filtered. The filtrate was diluted to 250 ml and aliquots containing from 229 to 291 micrograms of fluorine were pipetted into a Claissen flask. The test solutions were diluted with water to about 100 ml, acidified with 35 ml of sulfuric acid, and distilled at 135° C. The distillation temperature was controlled by adding water slowly from a dropping funnel during the test. The first 100 ml of distillate was collected separately, and from two to four additional 50 ml portions were collected. Fluorine determinations in the several portions of distillate from each sample given in table 1 show that the SPADNS-zirconium method can be applied to distillates, and that virtually all of the fluorine present was recovered by collecting 250 ml of distillate.
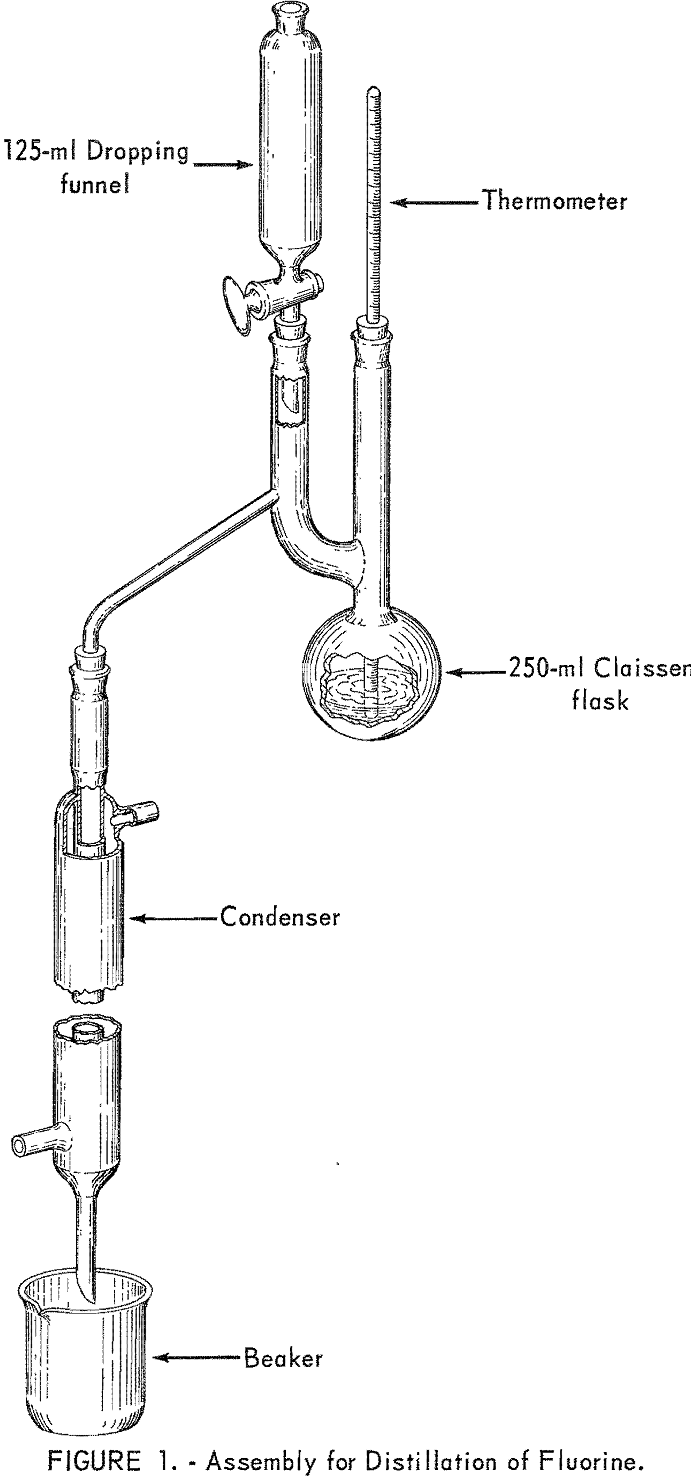
A relatively simple distillation apparatus, shown in figure 1, was employed. Experiments showed that one operator could carry out four distillations at the same time. Somewhat more elaborate apparatus with steam distillation also can be used for a fluorine determination.
Ashing with a Fixative Agent
Sodium Carbonate
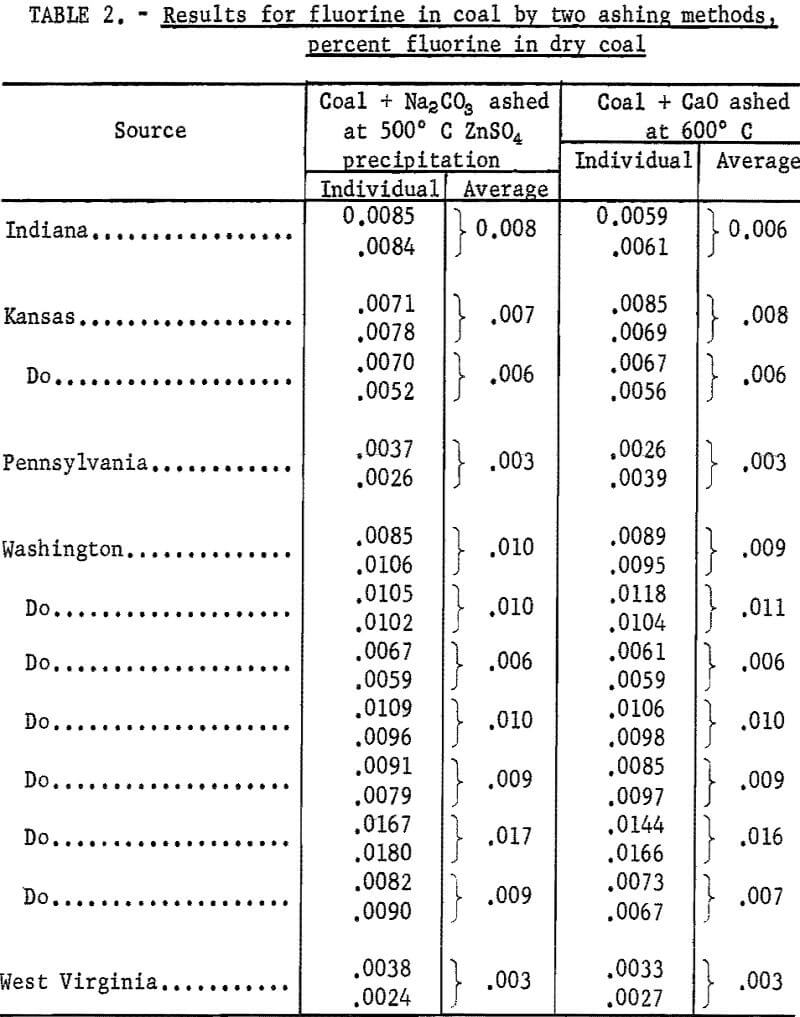
The method used by Kunstmann for ashing a coal sample mixed with sodium carbonate was tested. A 1-gram coal sample was mixed with 3 grams of sodium carbonate in a porcelain crucible, and the mixture was covered with 3 grams of the carbonate. After slow ashing at 500° C for about 16 hours, the residue was transferred to a platinum crucible and fused. The melt was leached with water and a zinc sulfate solution was added. This gave a voluminous precipitate containing most of the silica with zinc carbonate. After the precipitate was filtered and washed, the filtrate was treated with ammoniacal zinc carbonate solution and filtered again to complete the removal of silica. The final alkaline filtrate and washings were evaporated to about 100 ml, when the odor of ammonia was no longer perceptible. The solution was filtered and the combined filtrate and washings were transferred to a Claissen flask and were distilled with sulfuric acid at 135° C. Fluorine in the distillate was determined by the SPADNS-zirconium method and results are given in table 2.
Calcium Oxide
Plant materials such as leaves from corn or grass are ashed at 600° C for a fluorine determination after adding about 10 percent by weight of calcium oxide.
Tests were made on coal using calcium oxide instead of sodium carbonate as the fixing agent. Experiments showed that a 1-gram sample of coal mixed with 0.2 gram of calcium oxide in a 30 ml platinum crucible was ashed in about 3 hours at 600° C. Three grams of sodium carbonate were mixed with the ash residue and the mixture was fused over a gas burner. The melt was leached with water, filtered, and the residue was washed with a 0.1 percent solution of sodium carbonate. The filtrate was distilled with sulfuric acid and fluorine was determined in the distillate as before. Results for fluorine by the two ashing methods given in table 2 show that the more rapid CaO-ashing method is satisfactory for determining fluorine in coal. The results also show that a precipitation with zinc sulfate solution to remove silica is not required.
Distillation with Phosphoric and Sulfuric Acids
A report by Blake on refractory fluoride-bearing compounds and minerals showed that liberation of fluorine was complete in the presence of silica and aluminum when the distillation was made from a phosphoric-sulfuric acid solution of a sodium carbonate fusion of the sample. This modification was tested on coal samples ashed with calcium oxide and fused with sodium carbonate.
The melt was nearly all dissolved in a little water and 10 ml of phosphoric acid, then the solution and any undissolved material was transferred directly to the distillation flask. After 20 ml of sulfuric acid was added, the sample was distilled as before and fluorine was determined in the distillate.
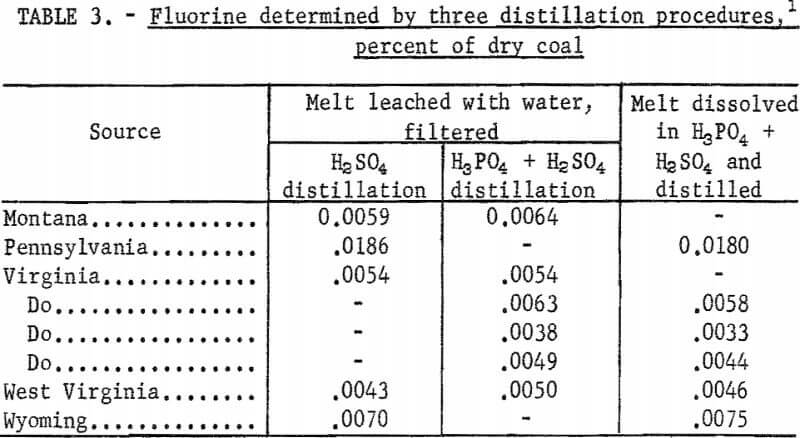
Results by this method are compared in table 3 with tests in which the sodium carbonate fusion was leached with water and filtered, and the filtrate distilled with sulfuric acid alone. In addition, a few tests were made in which the leached solution from a fusion was distilled with the mixture of phosphoric and sulfuric acids. The results for fluorine by these distillation methods showed no significant differences, and indicate that the water leaching and filtration steps can be eliminated. Some white precipitate, probably a calcium compound, formed during the distillations with mixed acids but this did not affect the recovery of fluorine.
Four experiments were made with spiked samples. Approximately 3 milligrams of opal glass (Standard Sample No. 91, 5.72 percent F) was added to 1-gram coal samples along with 0.2 gram calcium oxide. After ashing at 600° C the residues were fused with sodium carbonate. In the first two tests the melts were leached with water and filtered, then the filtrates were distilled with sulfuric acid. In the last two tests the melts were distilled directly with a mixture of phosphoric and sulfuric acids. Fluorine in the distillates was determined by the SPADNS-zirconium method. Results given in table 4 show that both methods gave about 95 percent average recovery of fluorine.
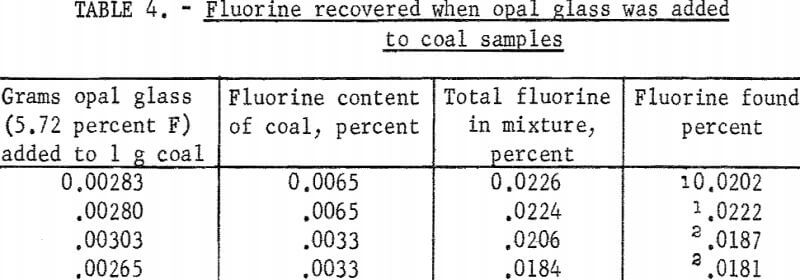
A method for determination of fluorine in coal based on these experiments is described in the appendix. Calcium oxide is mixed with the coal sample to retain the fluorine during ashing. Distillation of fluorine from an acid solution of coal ash gives a distillate that should be free of any interfering substances that may be present in the ash. A boiling temperature of 135 ± 5° C gives rapid volatilization of fluorine and a negligible amount of acid carryover in the distillate. With a glass distillation flask and glass beads to prevent bumping, the fluorine is volatilized as fluosilicic acid. Fluorine in this form is determined readily by the SPADNS-zirconium method.
Fluorine and Phosphorus in Float and Sink Fractions
Float and sink tests using mixtures of gasoline and carbon tetrachloride were made of three coals, and the moisture, ash, fluorine, and phosphorus were determined in the various fractions. The head samples ranged in fluorine content from 0.006 to 0.014 percent, and in phosphorus content from 0.010 to 0.068 percent. Solutions having specific gravities of 1.30 and 1.33, or 1.33 and 1.38 were used in separating each coal into two float and two sink fractions. These fractions with the head sample gave five portions for each coal having a wide range of ash content. The coals tested were a high phosphorus Tennessee coal, a West Virginia coal with medium phosphorus content, and a Pennsylvania coal with low phosphorus.
Yields of the float and sink fractions, and the ash, phosphorus, and fluorine contents of the fractions computed as percent of dry coal are given in table 5. Both the fluorine and phosphorus contents decrease as the ash content decreases, indicating that the fluorine and phosphorus in the coals tested are associated mainly with mineral matter.
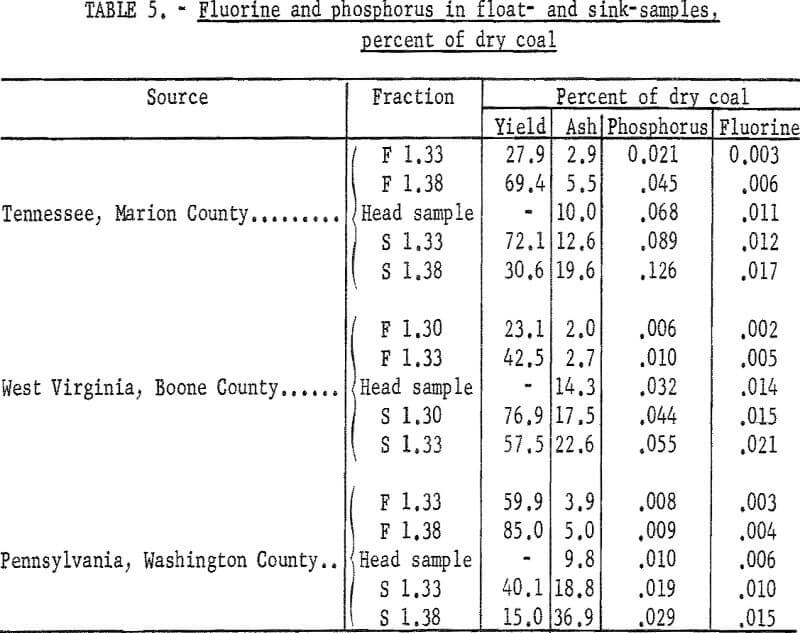
Crossley concluded that the fluorine mineral in British coals is a fluorapatite-type mineral that contains three atoms of phosphorus to one atom of fluorine. Although part of the fluorine in the coals tested may be present as fluorapatite, the West Virginia and Pennsylvania samples contain more fluorine than is required to satisfy this relationship, and the Tennessee coal contains less fluorine than is required. Probably the fluorine and phosphorus occur in other mineral forms in addition to fluorapatite. For example, the aluminum phosphate minerals evansite and wavellite were reported by Geer, Davis, and Yancey in shale associated with Washington coal.
Fluorine Determinations of Coals
A total of 83 coals was tested for fluorine content using the CaO-ashing method and distillation with either sulfuric acid or phosphoric-sulfuric acid mixture. Fluorine in the distillates was determined by the SPADNS-zirconium method.
The samples were collected from commercial shipments from the principal coal producing areas of the country. Data on phosphorus, chlorine, sodium, and potassium contents were reported previously. Because of interest in a possible association of fluorine and phosphorus, samples having a relatively high phosphorus content were tested first on the premise that these coals might have a high fluorine content. Fluorine was determined also in a number of low phosphorus coals. Duplicate tests for fluorine checked within 0.001 percent for about four-fifths of the samples and all checked within 0.002 percent. Average fluorine results (table 6) ranged from 0.001 to 0,019 percent. For convenience the phosphorus and ash contents are included in the table.
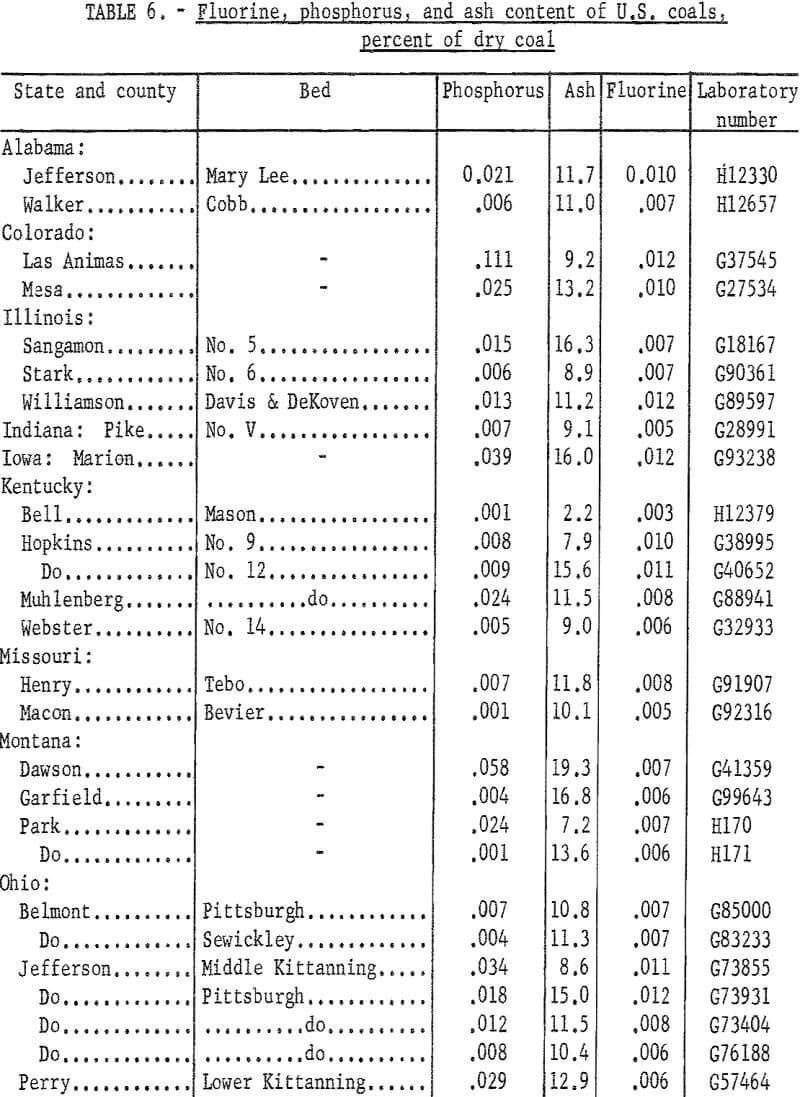
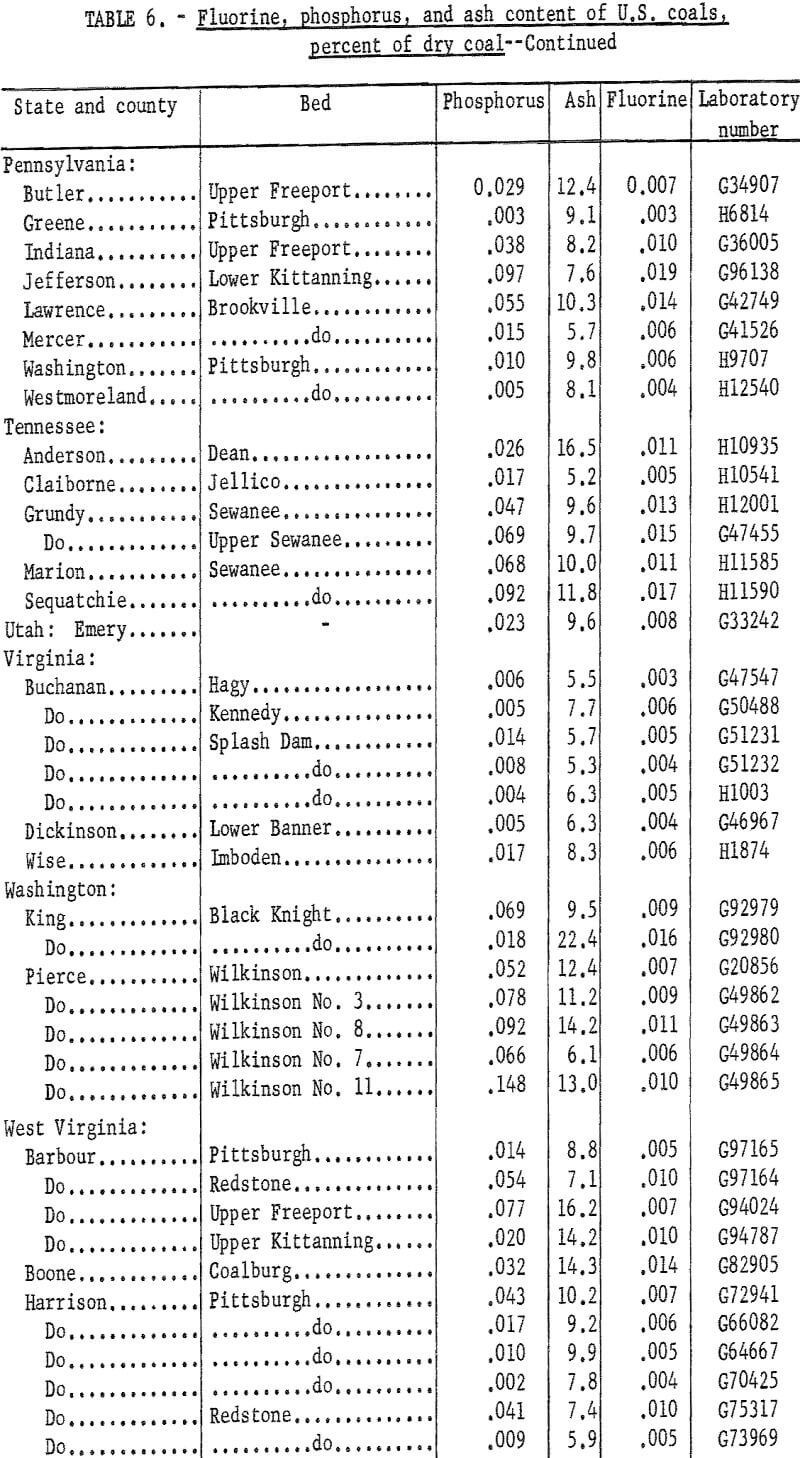
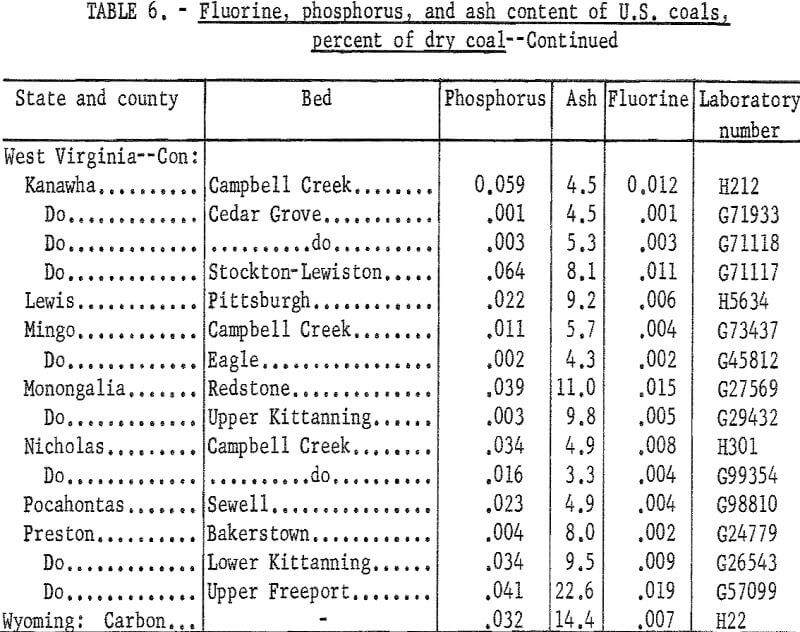
Fluorine in these coals could not be correlated with phosphorus content, although many of the coals with high phosphorus gave the higher results for fluorine. This is in agreement with results reported on Australian coals namely that in some coals most of the phosphorus and fluorine probably are associated in a fluorapatite-type mineral; in other coals only part of these elements occur in this form, and the presence of other minerals containing phosphorus or fluorine is probable.
Conclusions
- Calcium oxide can be used as a fixative agent for fluorine when ashing a sample of coal.
- A mixture of phosphoric and sulfuric acids is preferred for distillation of fluosilicic acid, but sulfuric acid also can be used alone.
- The SPADNS-zirconium colorimetric method is rapid, and can be applied readily for determination of fluorine in distillates.
- Tests of float- and sink-fractions showed that the fluorine was associated with mineral matter in the three coals examined.
- Fluorine and phosphorus contents could not be correlated, but coals having high phosphorus generally had the higher fluorine contents.
- Fluorine content of the commercial coals tested ranged from 0.001 to 0.019 percent of dry coal.
