Table of Contents
This article discusses the effect of physical and chemical modifications of starches on the anionic and cationic flotation of silica from oxidized iron ores and magnetite-taconite concentrates, and the results of the interaction of starch, pH, and calcium ions on the flocculation, clarification, and filtration of iron ore slimes and magnetite- taconite tailings. Starches, particularly when anionically modified, were found to be effective depressants in anionic silica flotation. British gums and dextrins were beneficial for oxidized iron ores, but none of the starches or starch derivatives appeared to have any effect on magnetite-taconite concentrates.
Anionic Silica Flotation
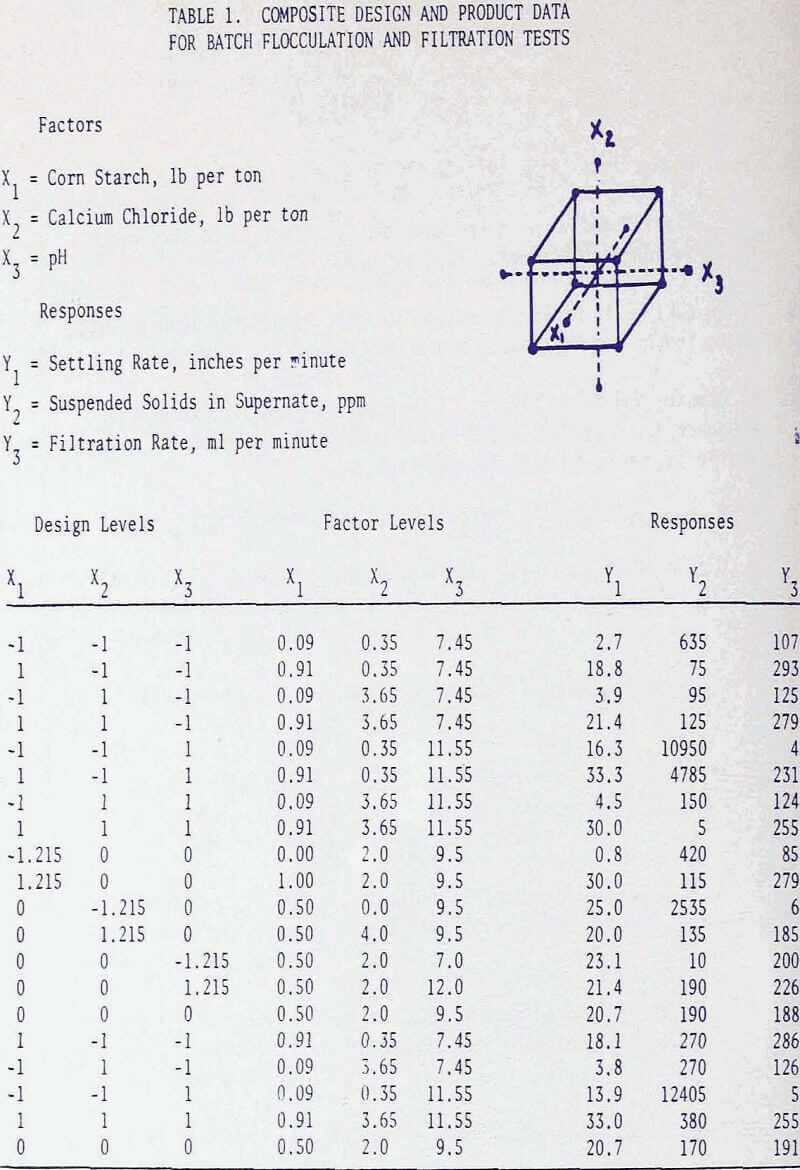
In the soap flotation of activated silica from iron ores a critical amount of starch addition exists beyond which flotation performance stabilizes. This critical amount of starch is dependent on the degree of grind and hence on the amount of slimes contained in the ore. With an ore sample essentially minus 325 mesh and containing approximately 50 to 55 percent iron, the critical amount of starch is about 4 lb per ton. This starch requirement contributes to a large portion of the total reagent cost, and it becomes of great interest to decrease this amount either through physical or chemical modification of the material.
It was noted in a previous article that by homogenizing a causticized solution the starch requirement could be greatly reduced. By analyzing the flotation pulp liquors for residual starch concentration and by comparing the iron recoveries, it became apparent that, whether the starch was used immediately as a causticized solution, or after homogenizing treatment, maximum iron recoveries were attained when the residual starch exceeded a certain concentration. It was presumed that a certain saturation coverage by starch was essential at all times during flotation, and that homogenizing reduced the molecular size. The starch thereby became more effective, weight-for weight, in closing the surface. In these tests the causticized starch solutions were homogenized in a Waring blender for 15 minutes. This homogenization time was selected because the causticized solutions of many starches reached nearly constant viscosity after 15 minutes.
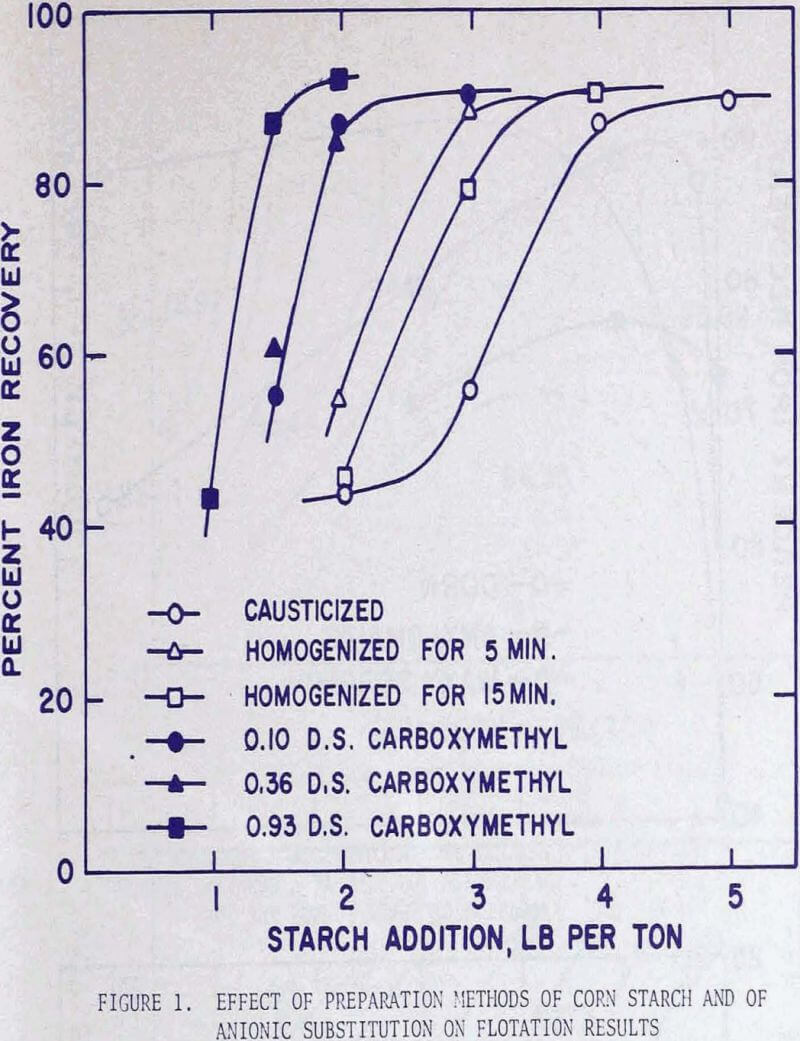
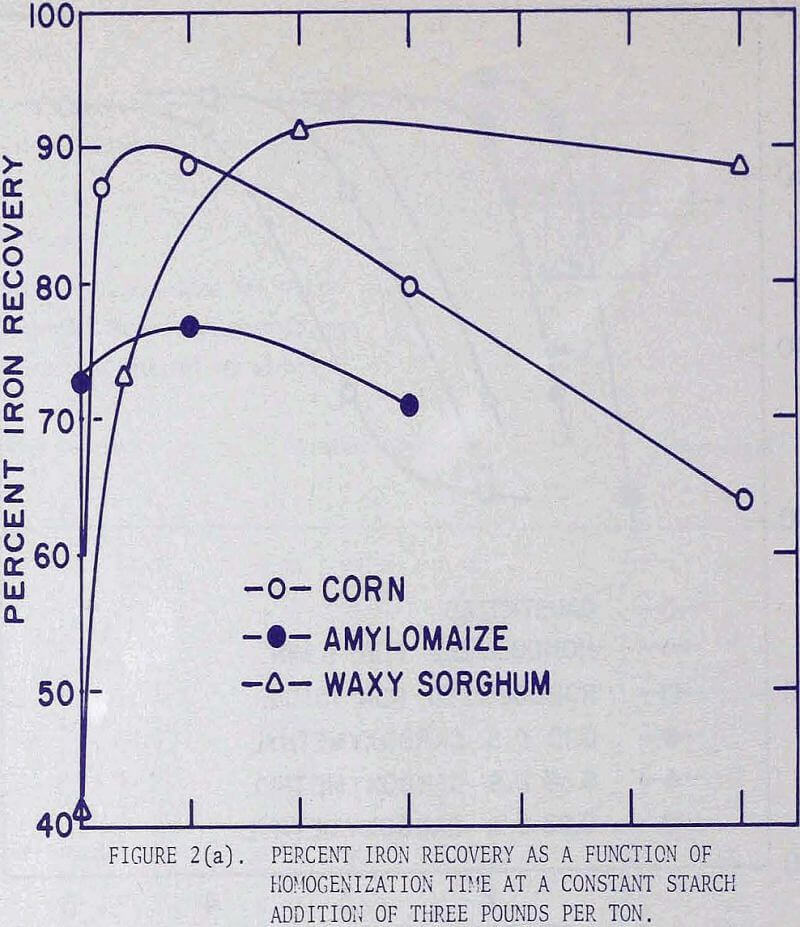
In order to investigate the effect of homogenization on flotation results,three series of flotation tests were performed using corn, waxy sorghum, and amylomaize starches. The experimental procedure was standardized in the following manner. The starch solution was prepared by mixing a 4 percent suspension of corn starch in distilled water with an equal volume of 1 N sodium hydroxide solution, followed by intense agitation in a Waring blender operated at 4000 rpm for a specified length of time.
For flotation the fine fraction of a Mesabi gravity concentrate analyzing 55.2% iron (corresponding to Ore C) was used. A 500-gram charge was pulped to 30 percent solids in a batch laboratory conditioner, a predesigned amount of starch was added, the pH of the pulp was adjusted to 11.8, and the pulp was conditioned for two minutes. This was followed by one-minute conditioning with 2 lb of calcium chloride per ton, and then two minutes with 0.8 lb of Acintol FA-2 per ton. The conditioned pulp was transferred to a Fagergren laboratory flotation cell, diluted to 20 percent solids, and floated for five minutes.
Since the grades of the concentrates remained virtually constant at approximately 62 percent iron, but the iron recoveries were in direct correlation with the amounts of starch addition, the graphical presentation of the flotation results was expressed in terms of iron recoveries.
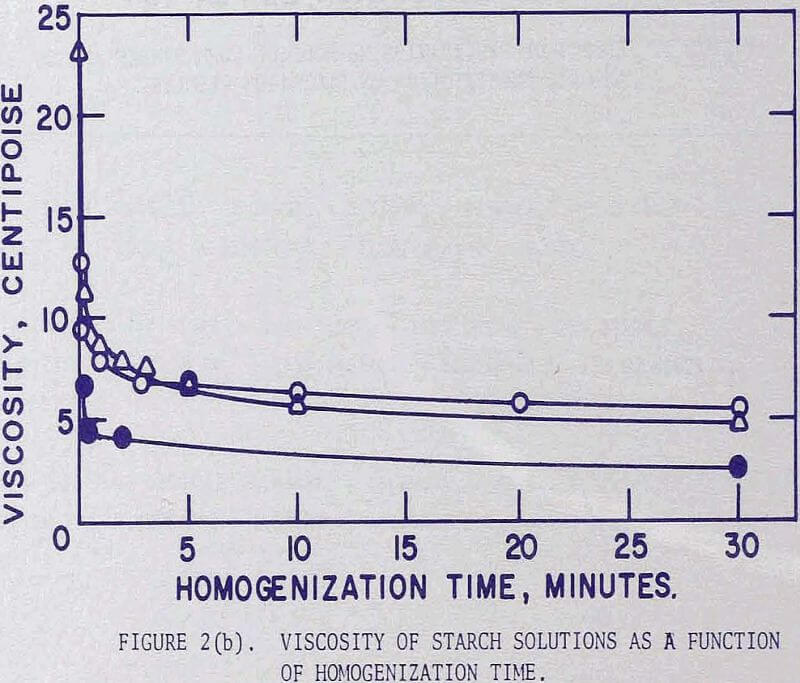
The change in the viscosity is greatest for waxy sorghum and decreases in the order of corn and amylomaize starches. It is interesting to note that the curves appear to be represented by straight lines when plotted on a log-log scale.
The adsorption of corn starch on hematite and quartz was shown to be strongly dependent on pH, and the adsorption densities under similar conditions were higher for hematite than for quartz. These phenomena were speculated to be due at least partially, to the interaction of the electrochemical conditions of the mineral surfaces and starch molecules. For oxide minerals, the hydroxly ions (and, accordingly, the hydrogen ions) are identified as potential determining ions, and therefore control the interfacial electrical condition. Common starches are known to be negatively charged over a wide range of pH in water.
Cationic Silica Flotation
The cationic flotation of silica from iron ores has received particular attention at the Mines Experiment Station for two purposes: (1) reduction of the silica content of magnetite-taconite concentrates from 8 or 8.5 percent to about 5 percent, and (2) upgrading of the combined concentrates from low-, intermediate- and high-intensity magnetic separators for the treatment of oxidized taconites, analyzing approximately 45 percent iron.
Since amine collectors are not particularly selective in floating silica from iron ores, a suitable iron oxide depressant is required. A considerable amount of work has been done in the past and, based on these results, British gum has been used extensively at the Mines Experiment Station. Although British gum was found to be fairly effective on oxidized taconites in increasing their iron recoveries, it is totally ineffective, and sometimes even deleterious, to the cationic flotation of silica from magnetite-taconite concentrates.
The effect of various starch derivatives on the beta-diamine flotation of siliceous gangue from oxidized iron ores was tested, and a number of the starch derivatives were found to act satisfactorily as depressants for iron oxides. In particular, British gum 9084 and dextrins appeared to give the highest selectivity, as was found by previous investigators. Chemically modified British gums, both anionic and cationic, were not as effective as depressants.
Magnetite-Taconite Concentrate
Much of the siliceous gangue in magnetite-taconite concentrates is present as locked particles with magnetite in different proportions in the coarse fractions, say plus 500-mesh. The flotation of coarse middlings with a cationic collector results in an indiscriminate flotation of fine magnetite, thereby affecting the iron recovery, oftentimes rather seriously.
A number of starches and their derivatives were tested as gangue depressants during the upgrading of magnetite-taconite concentrates by flotation. A 600-gram (dry basis) sample of a wet concentrate analyzing 64.2% iron, and having a size consist of 92% passing 325 mesh, was placed in a Denver cell and diluted to 25 percent solids. First, a starch solution was added to the cell and conditioned for one minute. Then Armac C was added and conditioned for another minute. Finally, one drop of pine oil was added and the pulp was floated for five minutes. The starches were solubilized either by autoclaving at 120°C, or by causticizing and homogenizing as before. The starch derivatives were solubilized by first dispersing them in water and then boiling the dispersion.
Flocculation and Filtration
The use of starches as flocculants was investigated in three different areas: the flocculation of an iron oxide slime, the selective flocculation and partial upgrading of a ground oxidized taconite crude, and clarification tests on the supernatant water from magnetite-taconite tailings. The effect of starches on the filtration of the semitaconite slime was also included to compare the factors governing both flocculation and filtration behavior of ore pulps.
From a few preliminary tests the settling rate, the clarity of the supernatant water, and the filtration rate were found to be complexly dependent on the levels of calcium ion, either as lime or calcium chloride, and of starch addition, and the pH. To investigate the effects of the three variables on flocculation and filtration of an iron oxide slime, a three-factor orthogonal composite design was used and the experimental points were fitted with a second-order equation by the method of least squares. For comparison, identical levels of the variables were chosen for both flocculation and filtration tests.
After the settling test, approximately 300 ml of the supernatant water was siphoned out and some of the solution samples were centrifuged at a speed sufficient to remove suspended solids without appreciably sedimenting the starch. The concentration of starch in the solutions was determined colorimetrically and the concentration of calcium ion by a complexometric titration method. The amounts of the suspended solids were determined by pipetting 100 ml from the well-agitated supernatant solution, evaporating to dryness, and weighing the residue. The weights were corrected for soluble salts by comparing the dry weights with equal volumes of the respective solutions filtered to remove the suspended solids.
The amount of suspended solids are seen to depend on the levels of both calcium chloride and starch addition, and there is an optimum combination of these two reagents varying considerably with the pH. At higher pH values the reagent requirements are higher, but it should be noted that an excessive addition is detrimental to the clarification of the supernatant water. The increase in turbidity with starch at constant calcium chloride addition and pH has been attributed to the protective action of the organic flocculant.