Because of the extensive surface area of oil droplets in emulsions, emulsion flotation offers possibilities for the recovery of finely divided mineral particles. Surfactants must stabilize the emulsion and possess an affinity for the desired minerals. Other important factors are the nature of the emulsion ingredients, the relative volumes of the continuous and discontinuous phases, the charge sign borne by the droplets of the oil phase, the zeta potential of the desired mineral, the soluble salt content the water-pulped and ground ores. At or near the isoelectric point or ZPC, the double layer about, the mineral-laden oil droplets is reduced in thickness and the droplets will coalesce and continue to aggregate into agglomerates. Inversion from O/W to W/O can occur depending upon a number of interrelated factors. Electrokinetic Instrumentation to measure, the ZPC, electrophoretic mobility, zeta potential and streaming current are invaluable in the application of emulsion flotation to metallic and no metallic minerals.
Background
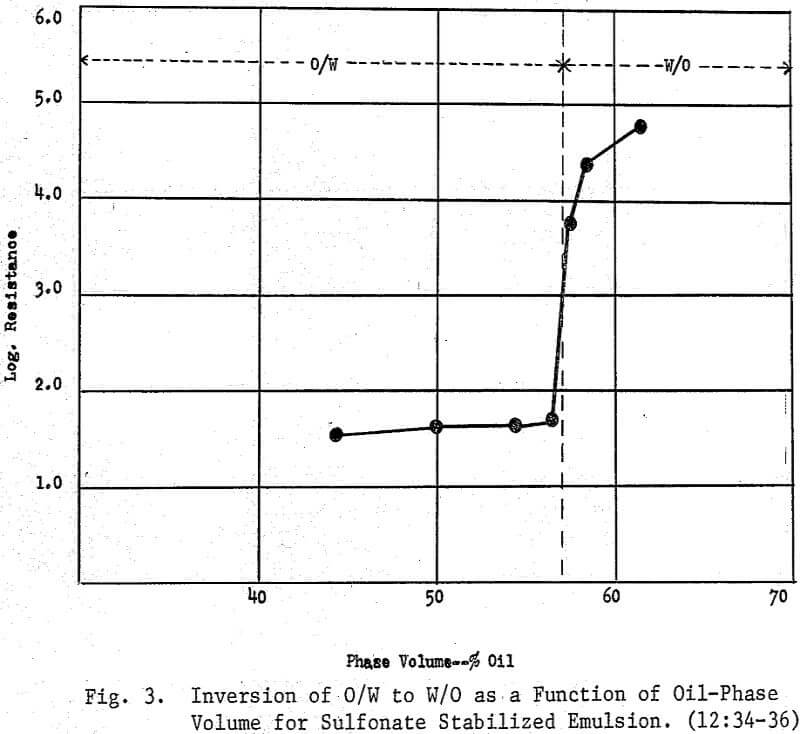
In mineral separation, an emulsion is a mixture of two immiscible or partially miscible liquids and one or more surface active agents, one of which may or may not be a collector. When the continuous phase is water and the dispersed phase is a ‘neutral oil’ (petroleum hydrocarbon), the surface active agent (or agents) will usually produce an oil-in-water (O/W) emulsion. The O/W emulsion is now in the form of fine droplets bearing a specific charge which may be either negative or positive depending upon the surfactant employed. Whether anionic or cationic, these reagents have two functions which are (1) to stabilize the emulsion by acting as emulsifiers and (2) to act as a surfactant possessing a specificity toward the desired mineral. Also, the mineral solids may further stabilize the emulsion or bring about an inversion from an O/W emulsion to a W/O emulsion (1:125) .
Invariably, the logic for emulsion flotation derives from von Reinders’ (2:127) analysis of Young’s equation. This equation for the wetting of solids by liquids is
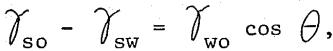
where θ is the contact angle and rso, rsw and rwo are the interfacial tensions of solid-oil, solid-water, and water-oil respectively. von Reinders pointed out three situations which are as follows:
- If rso>rwo + rsw, the solid particles will remain dispersed in the water.
- If rsw>rwo + rso, the solid particles will remain dispersed in the oil, and
- If rwo > rsw + rso, or if none of the interfacial tensions is greater than the sum of the other two, the solid particles will concentrate in the water and oil boundary. When the solid particles collect at the liquid-liquid interface, Young’s expression can be applied to give the following:
(a) If rsw < rso then the cos θ is positive and θ < 90°, which results in a major portion of the solid particles remaining in the water.
(b) If rso < rsw, cos θ is negative and θ > 90° which results in a major portion of the particles remaining in the oil; and,
(c) Obviously, there exists the unlikely situation when the contact angle is 90°, at which the solid particle is equally wetted by both the oil and the water.
At the outset in applying emulsion flotation, contact angle relationships are helpful, but electrokinetic measurements are often definitive and quantitative. Zero-point-of- charge (ZPC) and the relative magnitude (or lack of magnitude) of zeta potentials reflect on stability, coalescence, and inversion of an emulsion system in which a particular mineral is caused to participate. Consequently, electrokinetic instrumentation to measure the ZPC, electrophoretic mobility, zeta potential, and streaming current is invaluable in application of emulsion flotation to metallic and non-metallic minerals.
Breaking
When emulsions break, it is immediately apparent because the pulp becomes substantive—oil layers out at the water-air interface. Inversion and breaking are interrelated. Both of these phenomena involve flocculation and coalescence. On inversion of an O/W emulsion to a W/O type, the oil becomes the continuous phase, and the water forms droplets. This is a reversible process. Breaking occurs as a result of a merging of the droplets into a bulk phase. It is an irreversible process. On breaking, the stabilizing surfactants may remain in the water phase.
In emulsion flotation, breaking can be due to either mechanical or chemical factors. With relatively unstable emulsion, gravitational forces as intensified by mechanical agitation may cause breaking. Then, too, the impingement of the not-to-be-floated minerals against the droplets may be influencing. The soluble salt content of the fluid phase can supply cations of high valence which may promote breaking. Na+ and Ca+ ions so frequently present in the water phase may often be responsible for two bulk phases. Also, the weight content of the to be floated mineral in the pulp, the size distribution of the desired mineral, the percentage composition of the emulsion ingredients, and the quantity level of emulsion can be influencing factors. In fact, emulsion systems containing an extremely high quantity of oil are subject to breaking.
Reagents
By emulsion ingredients, it is meant the oil and the stabilizing surfactants; water is the usual continuous phase in emulsion flotation. Emulsion ingredients act synergetically to stabilize the emulsion, influence the degree of stability required, promote the reaction between the dispersed droplets and the desired mineral, and, in proper proportion, provide the desired flotation characteristics. For either nonmetallic or metallic flotation, usually two or more ingredients are required. It is exceptional when only one ingredient provides the requirements for an emulsion in flotation—especially when processing the finer fractions of water-pulped ores.
The ingredients for emulsion flotation can be selected from a wide variety of reagents. At least one of the ingredients in a multiple system must be surface-active; that is, the stabilizing or emulsifying agent must provide a surface-active group at the ‘neutral’ oil interface. The droplets of the oil-in-water dispersion will bear an electric charge. Ingredients, depending upon selection, can impart to the dispersed droplets a negative or a positive charge. Anionic surfactants for stabilization and the other previously listed requirements can be selected from salts of carboxylic acids, sulfates, phosphates, thio compounds, alkane sulfonic acids, and alkyl aromatic sulfonic acids, many of which are well-known collectors in the froth flotation.
In emulsion flotation, the quantity of emulsion ranges from several hundredths of a pound per ton of prepared-emulsion to several hundred pounds per ton. Usually, application to fine particulate sulfide ores requires relatively small quantities. On the other hand, such non-metallic ores as phosphates and manganese oxides require rather amazing quantities of emulsions. Apparently, the reasons for such quantities are the fineness of the particle size of the desired minerals, which is often an extreme, development in surface area, and the high elemental content of the ores, both of which result in heavy interfacial loads.
Reagent Recovery
Where the quantity of emulsion is large and the intrinsic value of the concentrates is low recovery of the reagents is usually an economic necessity. Solvent extraction methods ranging in yield from 87.8% to 95.7% of oil (diesel) and from 82.6% to 84.0% for fatty acids gave the following distribution of reagents in the products from processing both phosphate rock and manganese oxide ore:
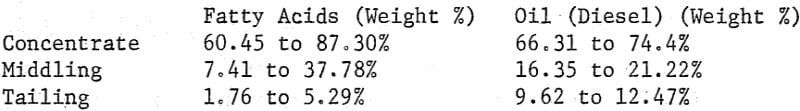
Obviously recovery of the reagents can be economically advantageous.
Where produced concentrates require calcining and nodulizing, the feed portion of a grate or grate-kiln system can be utilized as a steam distillation unit. Fumes or vapors from the wet concentrates are drawn off to a collector where condensation occurs. A manganese plant near Wickenberg, Arizona, used such a system and after adjustment in percentage composition the recovered emulsion was returned to the flotation circuit.