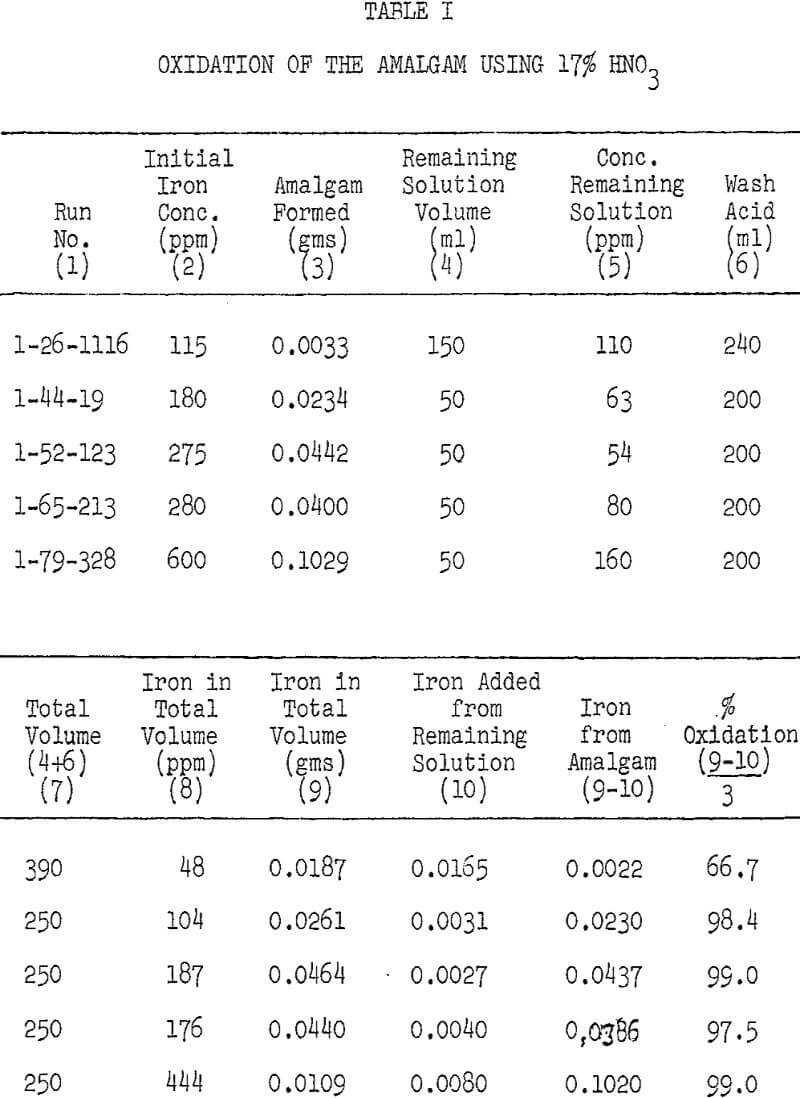
A study on the sulfuric acid extraction of alumina from some Pennsylvania ferruginous clays indicated that the published electrolytic method seems to be more efficient than the known chemical methods for removing iron from the leach liquor. The main disadvantages of the chemical methods are the consumption of excessive amounts of reagents and/or the precipitation of prohibitive amounts of aluminum. In the electrolytic method iron is deposited on the mercury cathode, while aluminum is not and remains in solution. This method, however, had been handicapped by the lack of an effective and economical way to purify the contaminated mercury cathode. .
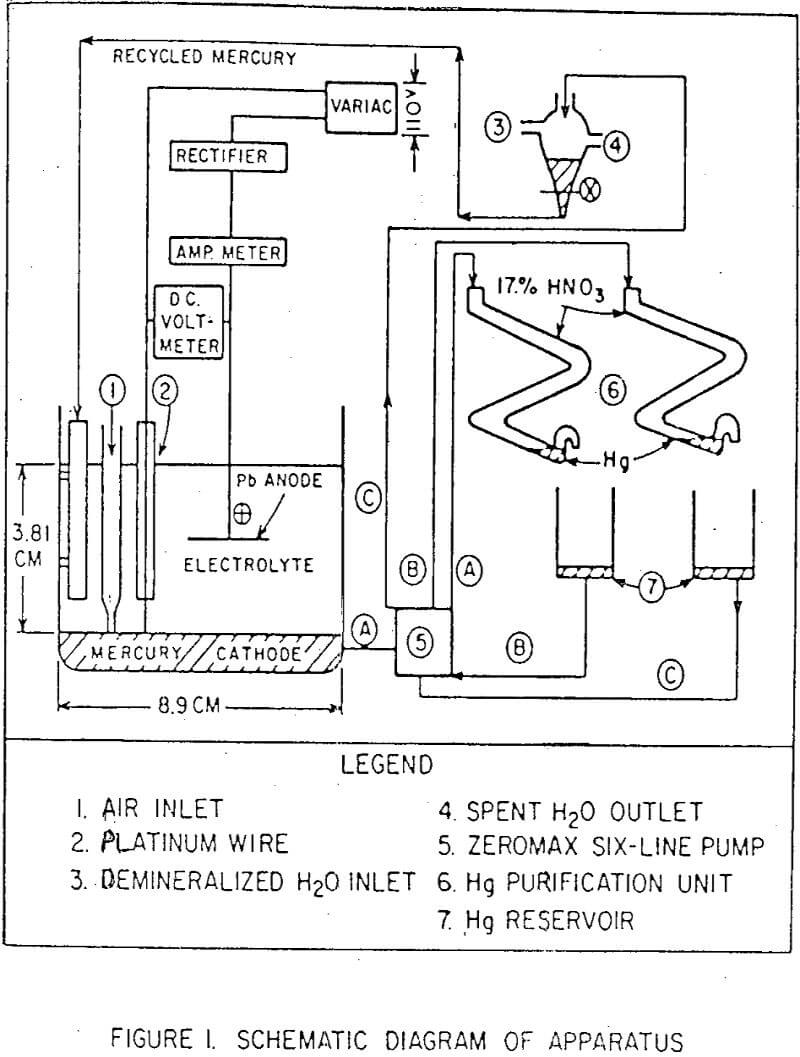
The electrolytic apparatus used in this investigation was designed and constructed to consist of an electrolytic cell and a mercury purification unit. The cell itself was a 600 mls. pyrex beaker with an outlet on the bottom and an inlet on the inside wall for recycling of mercury. The mercury cathode, having a surface area of 53.0 cm² was connected to the power source with a thin platinum wire, and agitated by air bubbling at a rate of about one bubble per second.
The mercury purification unit consisted of two verticle “M” glass tubes, with its lower ends fused separated onto two inverted “U” glass tubes. The M tube, having a constricted bottom of ¼” inside diameter, was filled with a nitric acid solution of 17% concentration. In order to decrease the possibility of mercury oxidation by the acid, mercurous nitrate, HgNO3·H2O, was dissolved in the acid to form a saturated solution.
Tests were performed to determine the effects of temperature on iron amalgamation. All the six tested electrolytes exhibit an increase in the percentage iron amalgamated with an increase in temperature up to 40°C, while other factors remain constant. A possible explanation is that an increase in temperature increases both the cathode current density (C.C.D.) and the thermal energy of the ions in solution.
Tests were also carried out to determine the influence of time on the removal of iron. Figure 12 shows that the iron amalgamated increases with an increase of time. Iron is practically removed from each of the tested electrolytes in about one hour’s time. A decrease of the potential from 5.0 to 3.5 volts increases the time for complete iron amalgamation almost eight-fold, i.e., from 37 minutes to 270 minutes.