Table of Contents
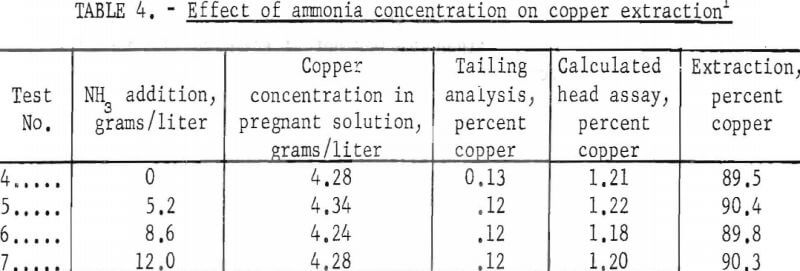
Under the conditions evaluated in this investigation, the extraction of copper from an argillaceous ore was not affected by the addition of ammonia to a cyanide solvent but was entirely dependent on the cyanide concentration.
A number of investigators have evaluated cyanide complexes as catalysts for the cyanidation of gold and silver ores. However, cyanide leaching of copper-bearing materials was not pursued until Leaver and Woolf made their comprehensive study of the copper minerals. Concurrently, Dunstan reported experiments on oxidized copper ores leached with an impure, low-cost cyanide and concluded that reagent consumption made the process uneconomical. In the period between these research efforts and the development of a more efficient cyanide regeneration system by Lower and Booth the cyanidation of copper-containing materials received little attention.
Technological advances, such as the improved method for regenerating cyanide solutions, necessitate periodic reexaminations of discarded and novel processes. Therefore, a literature survey of cyanidation processes was undertaken. It was found that copper minerals dissolve rapidly in the ammonium cyanide solutions applied to extract gold from ore. In this system, high-strength ammoniacal solutions are avoided, because the ammonia-copper-cyanide complex, 2CuCN·[Ca(NH3)4 · (CN)2, which forms, would redissolve and make the gold precipitation step more difficult. Since strong ammonia solvents dissolve the 2CuCN·[Cu(NH3)4]·(CN)2 complex and the CuCN, CuCNS, and Cu(CNS)2 precipitates that may form during normal cyanidation, the combination of ammonia and cyanide seemed to be applicable to the cyanidation of the argillaceous copper ore processed by the White Pine Copper Co., Ontonagon County, Mich. The Bureau of Mines at its Twin Cities Research Center, therefore, undertook a bench-scale investigation to determine if adding ammonia to cyanide leaching solutions would accelerate extraction of copper from the White Pine material.
Materials
Source and Characteristics of Ore Sample
A sample was obtained from the White Pine ore body located in the lower part of the Nonesuch Formation and the upper part of the Copper Harbor Formation. The sedimentary units of the ore body, listed in ascending rank are the Lower Sandstone, Parting Shale, Upper Sandstone, and Upper Shale. The mineralized zones occur in the Parting Shale and the lower 7 to 8 feet of the Upper Shale. The sandstone units contain very little copper, except in a small area near the White Pine fault.
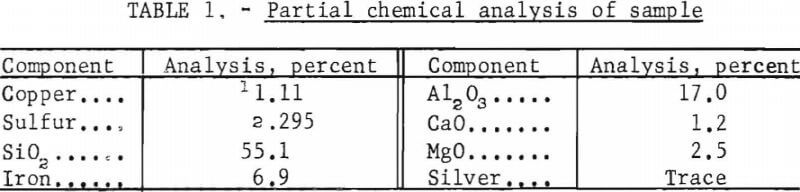
Chalcocite and native copper, the principal minerals in the ore body, are disseminated through the rock. The test sample contained an approximate 4:1 weight ratio of chalcocite to native copper, minor amounts of covellite, bornite, and chalcopyrite, and a trace amount of native silver. Segregation of flaky, metallic copper was an inherent characteristic of the ore and caused a variation in copper content among the samples. The gangue consisted mostly of argillaceous shale; however, some dolomitic material was present. Table 1 shows a partial chemical analysis of the sample.
Solvent
Solvents were prepared with 96 to 98 percent sodium cyanide (NaCN) and 28 to 30 percent reagent-grade ammonium hydroxide (NH3). For convenience in reporting, a 97-percent-NaCN purity is assumed, and the ammonia addition is based on a 28-percent-NH3 solution. The solvent was prepared by dissolving the NaCN in distilled water, adding the required volume of ammonium hydroxide, and diluting to 500 ml with distilled water.
Experimental Procedures
Minus 3-inch ore was air dried at room temperature, crushed to minus 8 mesh, thoroughly blended, and split into 800-gram charges which were stored in sealed glass jars. These precautions were taken to minimize sulfide oxidation which was believed to occur, superficially at least, during normal storage.
The minus 8-mesh ore was divided into 200-gram charges and ground in a laboratory ball mill. After the material was reduced to minus 65 or 100 mesh, it was filtered to minimize dilution and then mixed with the prepared leaching solution.
Standard leaching techniques were employed in which 500 ml of solvent and 200 grams of ore were contacted either by rolling in a bottle or by aerating in a modified 500-ml Buchner filter with a volume large enough to permit vigorous agitation. The rolling technique consisted of placing the solids in a 2-liter, small-neck bottle, adding the leachant left after washing the sample into the bottle, and then rolling the pulp at 72 rpm at room temperature, 22° C, for 4 or 24 hours. When the leaching cycle was completed, the slurry pH was measured, and the pulp was filtered. The solids were thoroughly washed with a 3-percent-NH4OH solution and then with distilled water heated to boiling. The pH of the diluted filtrate was adjusted with sodium hydroxide to the initial slurry pH and then analyzed for copper.
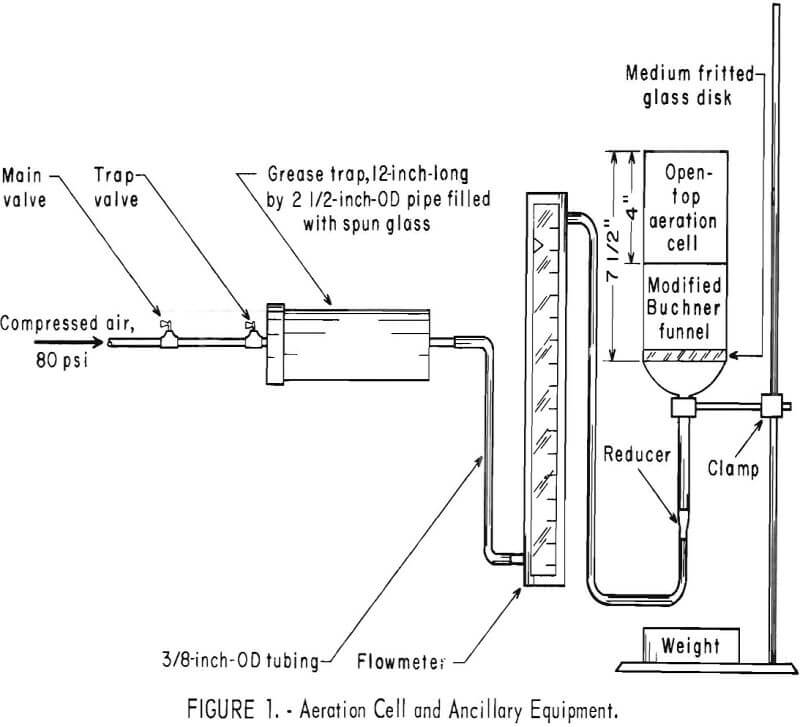
Figure 1 shows the equipment used in the aeration technique. The procedure involved putting the ore in the modified funnel, adding 500 ml of solvent, maintaining the airflow between 1.5 and 1.6 liters/min, and aerating the slurry for 4 hours at either 22° or 47° C. When the test was completed, the slurry was treated in the same manner as the tumbled pulp.
Results and Discussion
The effect of ammonia concentration on copper extraction from tumbled or aerated pulps, at room temperature or heated, was evaluated. An increase in slurry temperature should have given more favorable reaction kinetics however, the temperature had to be kept below 65° C to prevent excessive loss of ammonia. Also, aeration should have accelerated the dissolution of metallic copper and oxidized the sulfide minerals to more amenable forms.
Fineness of Grind
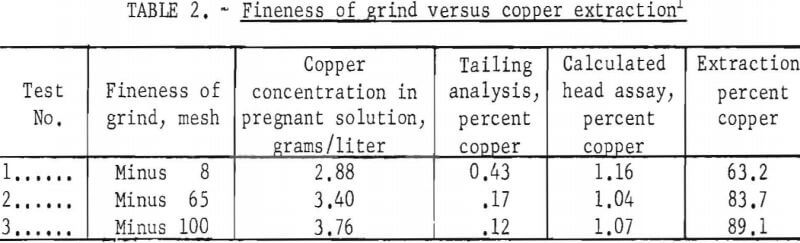
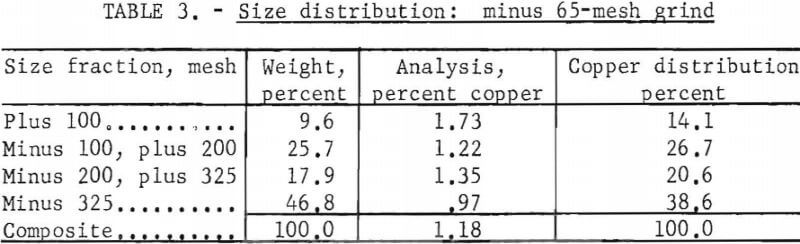
The effect of particle size was evaluated at minus 8-, 65-, and 100-mesh to determine the minimum grind required for complete extraction of the copper. Table 2 gives the pertinent test conditions and the metallurgical results. The data indicated that a minus 100-mesh grind should be used in the subsequent research. However, the pulp at this grind was extremely difficult to process; it filtered slowly and yielded a murky-gray, colloidal filtrate. Flocculants were not used to agglomerate the suspended solids, because previous experience had indicated that a flocculated pulp would entrap pregnant solution so that it could not be washed out. Therefore the minus 65-mesh grind was the practical choice, because at minus 65 mesh these problems were not encountered. The size distribution for the minus 65-mesh grind is presented in table 3.
Ammonia Effect
A series of tests employing additions of constant quantities of cyanide and variable quantities of ammonia was made using the rolling technique at room temperature; the results are reported in table 4. The ammonia concentration was varied from 0 to 12.0 grams NH3/liter but did not produce a significant change in the amount of copper solubilized. Since the copper extraction was approximately 90 percent ± 0.5, these tests indicate that the cyanide was the effective agent in dissolving the copper.
Pulp Aeration and Temperature
To determine if the effectiveness of the ammonia in the solvent could be improved, pulps were aerated m a modified Buchner funnel and evaluated at 22° and 47° C. The data obtained are summarized in table 5. A comparison of tests 8 and 9 shows that aeration had no noticeable effect on copper extraction.
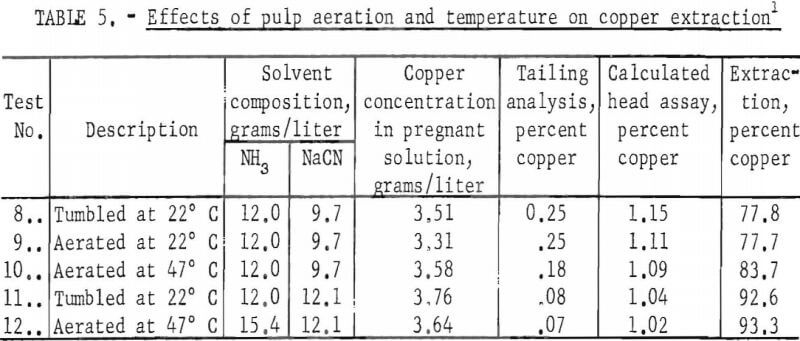
At a slurry temperature of 47° C, the effectiveness of an ammonia solvent containing 9.7 grams NaCN/liter was improved slightly; extraction was increased from 77.7 percent for pulp aerated at room temperature to 83.7 percent for pulp aerated at 47° C. However, at 12.1 grams of NaCN (tests 11 and 12), copper extraction was not significantly increased by heating the pulp to 47° C, which indicates that the increased recovery in test 10 was caused by increased cyanide reactivity. The increase in ammonia addition between tests 11 and 12 did not produce a significantly higher extraction thereby reconfirming that ammonia was not contributing to copper extraction. Further research on aerated, heated pulps was not carried out, because these slurries yielded approximately the same results as pulps rolled at room temperature.
