Table of Contents
The dump leaching of low-grade copper ores, as an integral part of the open-pit mining operations in the Southwest, has been practiced for the last fifty years and is increasing in importance as one of the major sources of copper. The recent acceleration in dump leaching can be attributed to the greater tonnages of low-grade ores mined and dumped at newer mines as well as resulting from increased stripping ratios at the older mines; to the relatively small investment in leaching facilities per pound of copper recovered contrasted with expansion of milling facilities to recover equal amounts of the metal by raising tonnage throughout; to low labor requirements needed for leaching; to the simple and continuous nature of the process needing little close supervision of trained operators; and, in recent years, to the awareness by the operators that dump leaching is not only an art but also a scientific process dependent on physical, chemical, and biological factors.
Materials and Methods
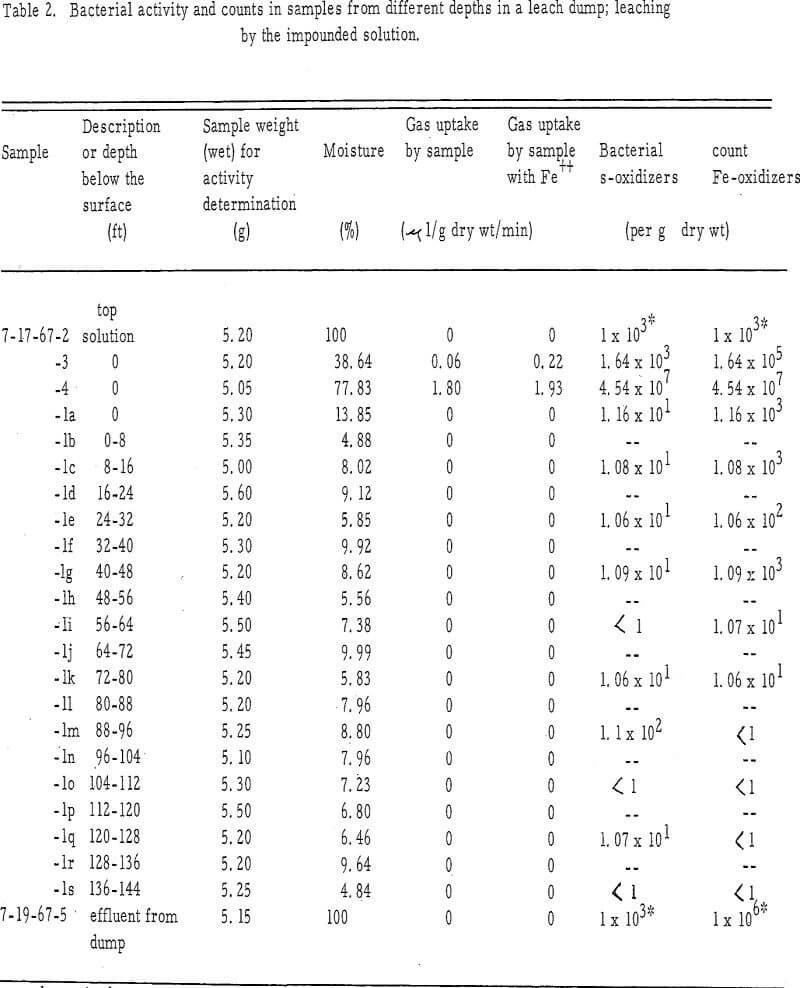
Samples for analysis of bacteria activity were collected from the mine dumps of the Chino Mine Division, Kennecott Copper Corporation, Santa Rita, New Mexico. All were placed in sterile 8-ounce screw-cap plastic or glass bottles and were returned to the laboratory facilities at Socorro the evening of the collection date. The samples were then stored at 3°C until analyses could be made.
To determine if the bacteria activity varied from the surface to the bottom of the mine dumps, samples were collected from 8-foot intervals in the dump, using a pneumatic Becker drill which removes a 3-inch-diameter core from an 8-foot interval and forces the sample up through the drill stem using 60 psi air pressure. Each 8-foot section of core was collected in a large pan, a sample from each core section being placed in a collecting bottle. No sample represents specific level within the dump, but rather, each represents an entire 8-foot section of a vertical column through the dump. The cores had a consistency varying from dry to moist sand. Each section of core was mixed during its accumulation in the collection pan.
Results and Discussion
The manometric technique used to determine the activity of bacteria was based on the utilization of oxygen during the oxidation of ferrous iron. The oxygen uptake from the samples showing bacteria activity was linear with time. Although the gas phase in the manometric reaction vessels was normal atmospheric air, the gas used was probably almost solely oxygen. Preliminary experiments in which 20 per cent KOH was placed in the center well of some reaction vessels to absorb all CO2 showed that no difference existed in the rate of gas uptake with or without the KOH. Although the chemoautotrophic bacteria also remove CO2 from the atmosphere, the gas was not a factor in these short-term experiments; it can be assumed that only the removal of O2 from the atmosphere was measured.
This indicates that thepotential for bacteria activity is present in the area but it lacks available ferrous iron for an energy source. In contrast, the rate of gas uptake in sample 7-17-67-4 was nearly the same for the sample with or without ferrous iron added to the reaction vessel (1.93 and 1.80-µl/g dry wt/min, respectively). Thus, this sample, taken from an area of a different leach pond, probably has an adequate amount of ferrous iron present as an energy scarce, since the addition of ferrous iron does little to increase the activity. The difference in activity noted for the two samples may be a function of the difference in microbial count.
It appears that there is little difference between the methods of impounding or spraying leach solution in terms of the zonation of biological activity. In both instances, the activity appears limited to the surface of the dump. The efficacy of the biological activity for the two methods of applying leach solution cannot be compared because of lack of sufficient data.
The bacteria activity may be located only on the surface because of the following: (1) The lack of moisture, which suggests the absence of sufficient leach solution to supply ferrous iron to establish a population of bacteria. The movement of solution through the leach dump has not yet been well defined. It is possible that the two sample cores may not have touched the major course of solution movement through the dump. Thus, the bacteria activity associated with the major solution course could have been overlooked. (2) The lack of oxygen within the dump could be a limiting factor. The chemoautotrophic bacteria, important for the leach process, are obligate aerobes and will be inactive in anaerobic environments. (3) The leach solution may become bacteriostatic as it progresses through the dump. Copper may accumulate to a bacteriostatic level.
Column Leach Studies
A series of column leach tests was conducted with ore from the surface of the dump and precipitation plant effluent liquors. These tests were designed to determine the effect of bacteria in oxidizing and hydrolyzing ferrous sulfate liquors in their passage through the bacteria zone near the top of the dump. Also, it was anticipated that some pertinent information on sulfide dissolution rates would be obtained from such experimentation.
In each column test, a prescribed volume of fresh precipitation plant effluent was percolated at a uniform rate through a bed of minus 1-inch ore. The column effluent solutions were measured and assayed daily for their content of total iron, ferrous iron, copper, and H+ (pH).
Relatively little copper was leached from the column by the ferric sulfate leach liquors. After the first few days, during which the copper content of the column effluent was abnormally high, the average increase in copper in the solutions amounted to 0.028 and 0.042 grams per liter at percolation flow rates of 0.0025 and 0.005 gallons per minute per square foot, respectively. The average retention times in the 3-foot column were 32 and 64 hours at the corresponding flow rates mentioned above. Thus, at the 0.0025 gallons per minute per square foot percolation rate and the optimum conditions of high ferric iron, acid, and contact in this column, a minimum of 48 days’ retention time and a dump 108 feet high would be needed to produce a solution containing 1 gram per liter copper.
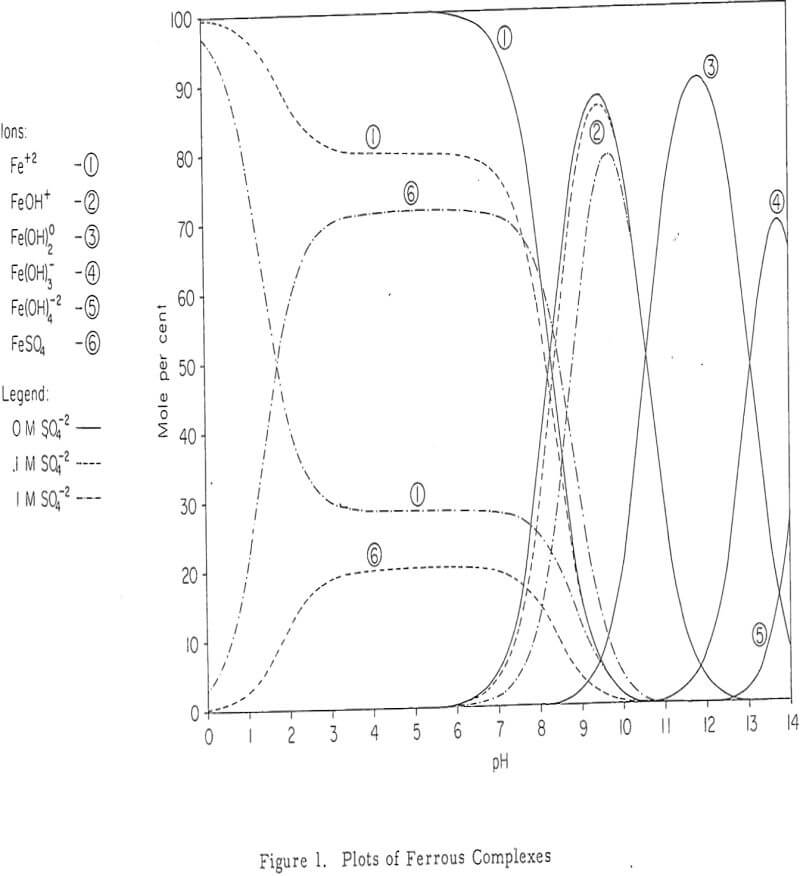
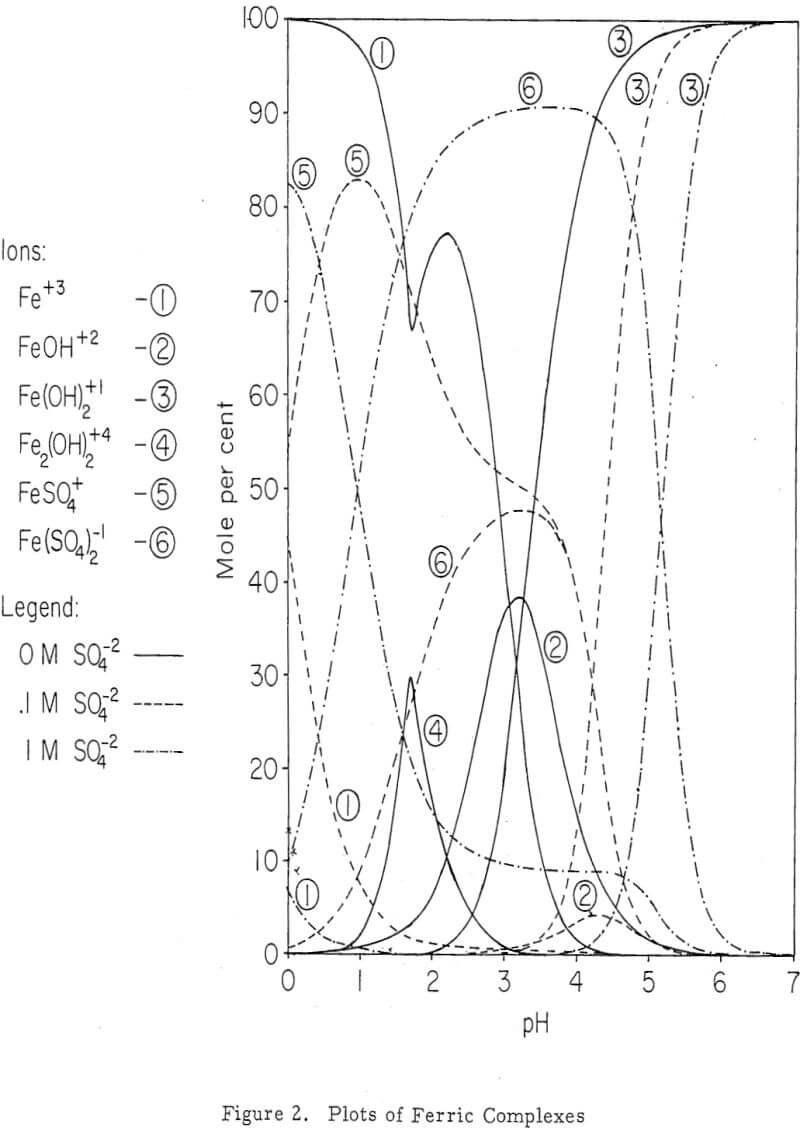
Nature of Iron Species involved in Dump Leaching
The interest in iron, as far as dump leaching is concerned, is twofold. Iron leach solutions may be harmful because of its tendency to precipitate under certain conditions. This may even extend to permanent sealing of the dump. On the other hand, iron especially ferric iron has been proved beneficial as an effective oxidant for copper sulfide minerals.
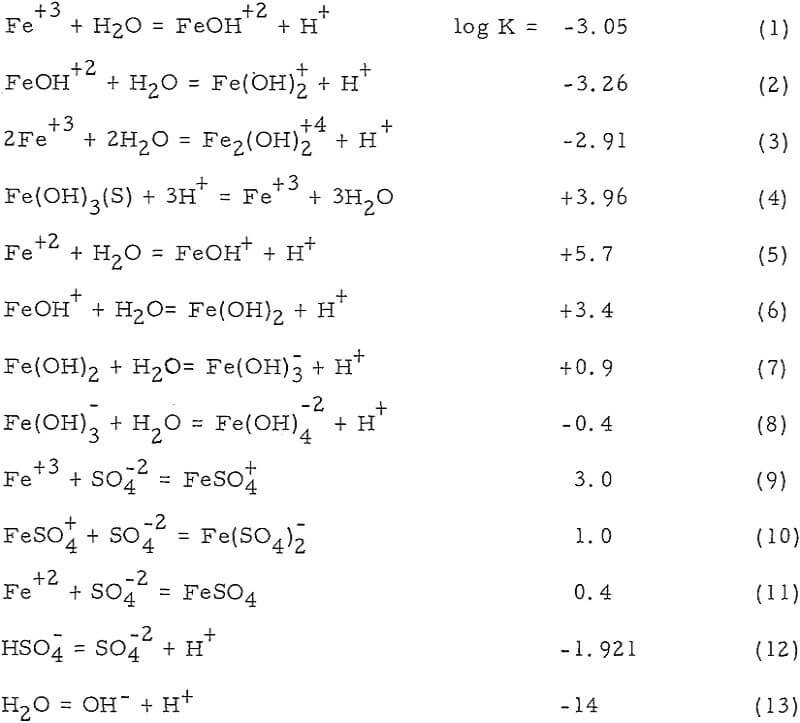
Study of the Chemical Composition of Dumps
Each of the 8-foot interval Becker drill samples was analyzed for moisture content, soluble salt content, the chemical composition of the interstitial solutions, and the chemical compositions of the rinsed and unrinsed solids. The following procedure was used to obtain these data:
- Each of the samples were crushed to minus 3 mesh and split into two 300-gram fractions.
- Moisture contents were determined by weighing, drying, and reweighing one of these 300-gram fractions. A total, copper analysis was run on the dried residue.
- The other 300-gram sample was rinsed on a Buchner filter with 50 milliliters of water or 900 milliliters of solution adjusted to pH 3.0 with sulphuric acid, dried, reweighed, and analyzed for iron, copper, and sulfur. The difference in the dried, rinsed weight of this sample, when washed with an excess of 3.0 pH solution, and the dried weight of the unrinsed sample was considered the weight of the soluble salts. The rinse solutions were analyzed for copper, total iron, ferrous iron and H+ (pH).
- In some instances, the minus 325-mesh fractions of the rinsed residue were weighed and assayed for copper, sulfur, and iron.
Iron Oxidation and Hydrolysis
The data obtained in this study suggest that iron oxidation and hydrolysis reactions occur in the upper parts of an active, permeable leach dump and are primarily a result of bacteria, air, and ground-water oxidation of a surface zone of retained iron salts. These reactions apparently occur during the interim periods between leach solution additions to the dump, as well as during the solution addition period.
The location of the iron oxidation and hydrolysis reactions apparently is in the upper regions of an active, permeable leach dump. Profiles of the concentration of the constituents (Fe+³, Fe+², H+ and Cu+²) of the interstitial solutions show a change from ferrous iron to ferric iron and acid near the surface. Iron and sulphur, which are constituents of the residual iron hydroxide product, likewise diminish in an exponential fashion with dump depth.
Copper minerals, particularly sulfides, maybe dissolved within a dump by several means: dissolution by a ferric sulfate-acid solvent, alternate wetting and drying cycles with plain water or solutions and air, electrolytic reactions wherein different metallic minerals and/or salts come in contact with one another and form small electrolytic cells, and dissolution by acid in combination with neutral salts, such as NaCl.
Although ferric sulfate and acid may be responsible for much of the leaching that occurs in a sulfide leach dump, there is some evidence that they are not responsible for all the leaching. For one reason, there is not sufficient under even the best of conditions to dissolve the amount of copper that is dissolved. Stoichiometrically, it requires 4. 34 grams of Fe+³ to leach 1 gram of copper from chalcocite and oxidize the sulfur that is formed.
The importance of alternate wetting and drying in dump leaching cannot be overemphasized. It is one of the major factors involved in the dissolution of copper sulfide minerals contained in the ore, especially in larger pieces and in the removal and crystallization of soluble salts at the surface of the ore particles through evaporation and capillary action. Besides alternate wetting and drying, undoubtedly diffusion plays an important role in dump leaching.