Table of Contents
The following conclusions are drawn from the results of the laboratory tests of amalgam electrorefining of zinc and tin:
- Amalgam electrorefining is a method by which these metals may be refined to total metal impurity concentrations of less than 5 ppm in zinc and 9 ppm in tin. The suitability of metal for amalgam refining depends on the solubility of the metal in mercury and the electrochemical relationships of the metal and the impurities at the amalgam anode and at the cathode.
- In the systems investigated no evidence was found of deterioration of amalgams, electrolytes, or addition agents any different from those encountered in the usual electrorefining and electrowinning practices. Several systems were operated for months with only normal makeup of addition agents and minor adjustments of pH and electrolyte components to compensate for drag out. No unusual requirements for materials entering the system were detected. Contamination of the zinc amalgam with several more noble metal impurities up to the limit of their solubility did not affect the performance of the amalgam or the purity of the cathodes produced.
- Mercury will be found in the cathodes in amounts of about 2 to 10 ppm unless provision is made to remove it from the electrolyte. Operating a cell at reduced temperature decreased, but did not eliminate, mercury in the cathodes. Circulating the electrolyte over tin turnings effectively reduced the mercury in tin cathodes. Heating zinc cathodes in vacuum effectively reduced the mercury in the cathodes. Each of these methods of control was not tried with the other metal.
This research was undertaken to investigate electrorefining of zinc and tin from a mercury amalgam anode as a method of producing high-purity metals. The purest base metals available have an impurity content of less than 5 ppm and command a premium of from 5 to 100 times the price of ordinary commercial grades. The research was concerned only with the problems associated with the preparation of high-purity metals and not their uses.
Other Bureau of Mines investigations have reported on the recovery of zinc from highly contaminated wastes in the galvanizing industry, the recovery of tin from the waste product of a tin smelter, and the recovery of byproduct cadmium in the electrolytic zinc industry.
Amalgam electrorefining is a process whereby a metal is dissolved in mercury, and the amalgam is used as the anode in an electrolytic cell. Rich amalgam is formed and circulated in an external circuit and flows by gravity into an electrolytic cell. The metal to be refined is anodically oxidized into the electrolyte and deposited by cathodic reduction from the electrolyte onto a cathode sheet. The value of the method takes advantage of the unique properties of a fluid anode in preventing the oxidation of the more noble impurities at this electrode.
One of the factors to be considered in an amalgam electrorefining process is the solubility of the metal to be refined in mercury as well as the solubilities of the impurities. Table 1 shows the room temperature solubilities of the more common metals that may be amenable to amalgam electrorefining and of the impurities which may occur in commercial grades of the metals. However, these solubilities may be affected by the presence of other metals. The effect of temperature on the solubilities of zinc and tin in mercury is shown in figure 1. This temperature effect is typical for other metals.
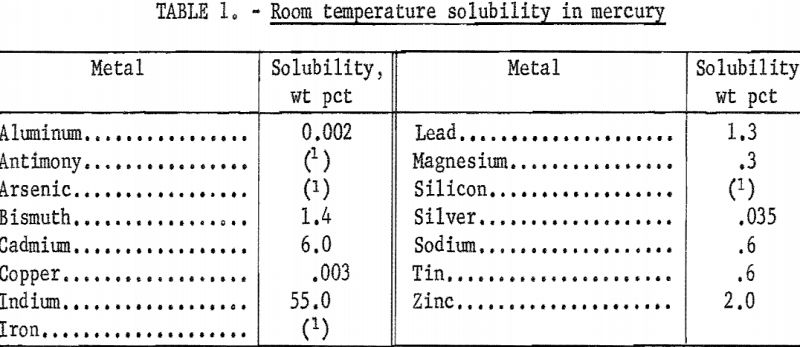
A second consideration is the electrode potential of the metal and of the impurities when dissolved in mercury. The values of the potentials of pure metals in amalgam solution as reported in the literature are in some disagreement, usually in the second decimal place. Approximate values are given in table 2. Even if these values were known within the usual limits of accuracy, other factors such as ionic concentrations in the electrolyte and formation of intermetallic bonds in the complex amalgams would have some effect on these values. As a general rule any impurity in an amalgam anode which has a less noble half-wave potential than the desired metal will be oxidized preferentially and enter into the electrolyte. If, on the other hand, the half-wave potential is more noble than that of the desired metal the impurity will be retained in the amalgam. Under these latter conditions the impurity will accumulate to its limit of solubility with the excess forming a slush that can be removed periodically. Discharge of the desired metal or of an impurity at the cathode will be dependent on the electrode potential of that particular species.

Procedure and Equipment
In order to take maximum advantage of the selectivity offered by amalgams, it is essential that a film of rich amalgam be continually exposed to the electrolyte at the anode face. This may be done by feeding amalgam to the top of a stationary vertical support sheet from an overflow weir. An alternate design is to use a rotating vertical support sheet, the lower portion of which is in an amalgam pool.
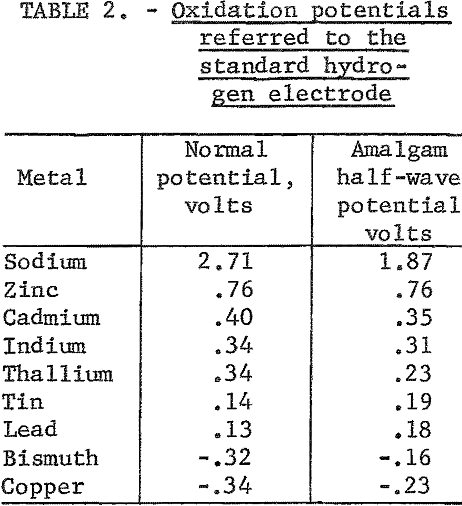
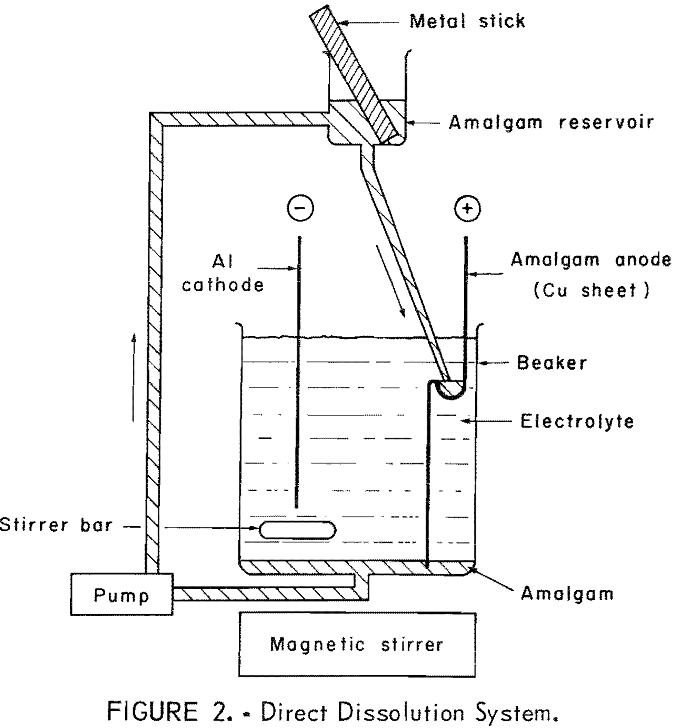
The amalgam may be formed and maintained by direct dissolution of the impure metal into the amalgam as depicted in figure 2. An alternate method is shown in figure 3, where the amalgam is formed and maintained by electrolytic means. In this method a second cell is used to anodically dissolve the metal and then deposit it electrolytically into an amalgam cathode. There is a theoretical advantage in depositing the metal electrolytically into the amalgam in that a typical electrorefining step is performed in the dissolution cell.
Most conventional electrorefining electrolytes, including the wide range of addition agents, should be suitable for use in amalgam electrolysis. Where the amalgam is formed and maintained by anodic dissolution, addition agents have no useful purpose and therefore are not required in the dissolution cell.
In the laboratory work, investigations of the effects of variables were made in small cells fabricated from 400-ml beakers. These cells used stationary anode support sheets constructed from thin gage copper which readily wet with mercury and kept a continuous amalgam film. Cathodes were made of aluminum sheet which provided good support for deposit growth and permitted easy removal of the deposits. Active cathode areas of up to 4 sq in. were obtained by masking off the unwanted areas. Primarily as a result of cell and electrode fabrication, the ratio of anode to cathode area was slightly >1.
Extended process life tests were also made using this type of cell. A larger cell with an electrolyte volume of 4.1 liters and a cathode area of 43.5 sq in. was operated to produce zinc in a routine manner for several months. In addition to the small cells, a rotating anode cell with an electrolyte volume of 750 ml and a cathode area of 6.3 sq in. was operated to produce tin for several weeks.
The metals, zinc, tin, and mercury used in these studies were selected from those readily available from commercial sources. Special high-grade zinc although obtained at a slight extra premium, was chosen as typical of the commercial supply. Results from analyses of lots from three different vendors are shown in table 3. Malayan tin was obtained from domestic suppliers capable of furnishing primary grades. The results of analytical determinations for the two samples used in this study are shown in table 4. Instrument grade mercury, certified to have a nonvolatile residue of less than 0.025 ppm. was used to prepare all amalgams.
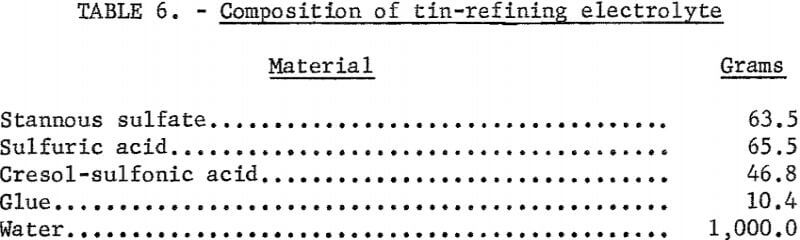
The electrolytes used in these investigations are similar to those used in past work and are given in tables 5 and 6. Distilled water was used to prepare electrolytes from commercially available reagents. Whenever possible, analytical reagent grade materials were used. These included ZnCl2, NH4 Cl, and H2SO4. Each had an undesirable impurity content of less than 50 ppm. The only grade of SnSO4 available for use was listed as “purified.” This was used without further treatment. The addition agents were also used without being purified. The electrolyte for zinc refining was selected
because of the excellent quality of the deposits obtained. Use of only 2 grams of glue in the zinc-refining electrolyte was preferred to the amount specified by Sullivan and Chambers; it required less conditioning time to produce cathodes of a good physical quality.



Electrolyte maintenance in both the zinc and the tin systems was relatively uninvolved. It required only additional glue makeup, when the quality of the cathodes indicated a need. All fresh glue additions required some conditioning before full cathode physical quality was restored. Small samples of electrolyte were withdrawn, when continued degradation of cathode quality was observed. Analyses of these indicated which chemical component was required to compensate for drag-out losses. The zinc system pH was maintained between 4.7 and 5.2, generally by addition of aqueous HCl.
The current density used for zinc deposition was usually 40 amp/sq ft. At this current density and with an electrode spacing of approximately ¾ in,, about 0.15 volt was required at normal room temperatures. Current efficiencies in excess of 98 percent were achieved. When electrolytic amalgam maintenance was used for zinc, the voltage drop across the dissolution cell was about 0.07 volt.
Rather than operate the tin cells with controlled current and a relatively stable cathode current density, it was necessary to operate with controlled voltage to prevent the codeposition of lead with tin. The maximum permissible voltage varied somewhat with the cell temperature, degree of electrolyte conditioning, and length of time the deposit had been forming but was about 0.2 (±0.05) volt with 1 in. electrode spacing at normal room temperatures. Under these conditions the current density varied between 10 and 16 amp/sq ft. Tin cathodes were obtained at current efficiencies in excess of 98 percent. When electrolytic amalgam maintenance was used the voltage drop across the dissolution cell varied from 0.07 to 0.10 volt depending on the current flow through the system. Provision was made to circulate the electrolyte through a fiberglass filter to remove precipitated lead sulfate, thus minimizing the chance of mechanically occluding PbSO4 in the tin deposit.
Results and Discussion
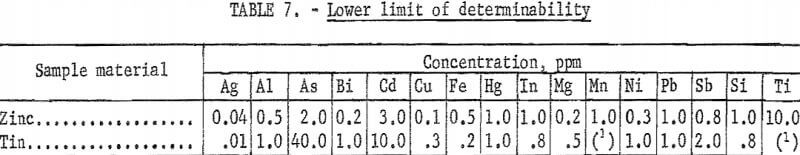
All analyses were made with the arc spectrograph using a method developed at the Bureau’s College Park Metallurgy Research Center specifically for this work. Table 7 lists the lower limit of determinability for various impurities in zinc and tin. This limit has been defined as that concentration which will produce a spectral line 5 percent (transmittance units) above the surrounding background. The limit of detectability, a value below which an element would be reported as “not detected,” is believed to be approximately 10 percent of this. All results reported are the averages of three determinations made on each sample. Since the method was intended to give maximum sensitivity, very high values for some impurities in the starting materials may be somewhat distorted.
A summary of the analyses of the amalgam-refined zinc cathodes produced is given in table 8. The results for cathodes from the small cells and from the large cell are tabulated separately as there are some differences. Values which departed considerably from the usual grouping of results are shown in separate columns in the table. Small differences between numbers have little significance, since the precision of the method at the limit of determinability was reported to be 28 percent.
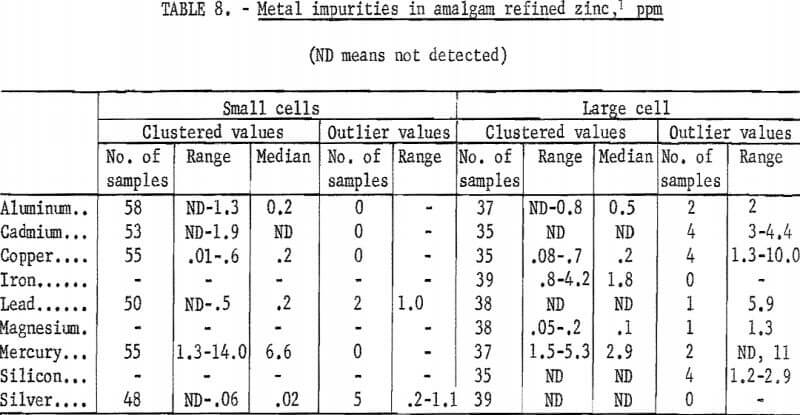
The concentrations of metal impurities in the cathodes produced in the large cell were generally lower than in the cathodes produced in the small cell. There is no apparent reason for any difference. Other than mercury, aluminum and iron were the major impurities found. Copper was detected in all samples. Median values for cadmium and lead were very close to the limit of detectability, and silver was found on occasion in the hundredths of a part per million range. Mercury was found in amounts of from 2 to 10 ppm.
A series of tests was performed in duplicate wherein cadmium, lead, and silver were deliberately added to the amalgam in increments until the amalgam was saturated with these impurities. No increase in contamination of the cathodes was found.
A series of cathodes was made with the cell maintained at reduced temperatures, down to 5° C, to determine the effect of cell temperature on the mercury content of the cathodes. The results were; 15° C-6.0 ppm Hg; 10° C—4.2 ppm Hg; and 5° C— 1.5 ppm Hg. While there was a reduction in the mercury content of the cathodes as cell temperature was reduced, the lowest mercury contents achieved were not sufficiently low to accept this as a method of “eliminating” mercury contamination.
Mercury was effectively removed from cathodes to below the limit of detectability by heating the cathodes in a vacuum. Sufficient tests were made to establish the effectiveness of the method, but no attempt was made to establish the time, temperature, degree of vacuum, or thickness of cathode relationships.
The results of the tests of amalgam refining as a method of producing high- purity tin are summarized in table 9. Cathodes were made with and without electrolyte purification by circulation over tin turnings and with and without controlled voltage. These changes in methods of operation affected the amounts of impurities found in the cathodes except for aluminum, iron, magnesium, and silicon.

The first tests made in the production of high-purity tin cathodes were made with constant current and with the electrolyte circulated externally through the fiberglass filter medium to remove precipitated PbSO4 but without other electrolyte purification. Analyses of the cathodes showed very nigh and erratic results for lead. The mercury and copper contents were comparable to those realized in the zinc test work. Six of the 24 tests showed high nickel content in the cathodes.
A tube containing tin turnings was then inserted after the fiberglass filter in the electrolyte circulation system. The copper and mercury in the cathodes were reduced, in most cases, to below the limit of detection. This method of reducing the copper and mercury content of the cathodes was not tried in a zinc system.
As noted in table 2, both the normal electrode potentials and the amalgam half-wave potentials of tin and lead are very close to the same values with lead slightly more noble in each case. In this application, with an electrolyte relatively concentrated in tin and the solubility of lead in the sulfate electrolyte very low, lead in the amalgam has a less noble potential than tin. As a result, lead in the amalgam will be oxidized before tin and will enter the electrolyte. The excess lead ion over the solubility of PbSO4 in the electrolyte will be precipitated, thus maintaining the lead roughly 0.2 volt less noble than the tin. These conditions would favor deposition of tin at the cathode. Thus, instead of operating at constant current, if the voltage across the deposition cell was held to about 0.25 volt, the lead in the cathodes was reduced to less than 5 ppm. However, a lead content less than 1.5 ppm was never achieved, with the expected value between 1.5 and 5 ppm.
