Table of Contents
The exploratory tests conducted with activated carbon and a ferruginous acid mine water indicate that ferrous iron oxidation takes place catalytically at an extremely rapid rate. The brevity of this study did not allow defining the reaction kinetics, or resolving the various factors affecting the activated carbon-air-acid mine water system.
The favorable preliminary results warrant further investigation to determine the efficiency and effective life of the carbon in a continuous flow system; the constraints that limit the effectiveness of the carbon; whether acid regeneration will restore the carbon to its original high efficiency if the surfaces become fouled with solids; and what cost and performance standards need to be established, provided the carbon-air-acid mine water system is effective.
A rapid oxidation scheme such as the carbon-air-acid mine water system to convert ferrous iron to ferric iron prior to neutralization would greatly facilitate the mine water treatment process. The use of catalytic oxidation would provide distinct advantages. With all of the iron in the ferric state, the neutralization reaction would be characteristic of a strong acid and any base. This suggests the application of a low-cost alkaline agent, such as limestone, which would provide the necessary basicity to neutralize all of the acid and precipitate all of the iron and aluminum. Stoichiometric relations can be precisely controlled at a pH level which would insure optimum use of alkaline agent. Aeration, of course, would no longer be necessary. A more compact and reduced volume of sludge produced at near neutral pH conditions would significantly reduce sludge separation and sludge handling costs.
When the Bureau of Mines, in 1966, as a special adjunct to its comprehensive research program on the chemistry of mine water, undertook a short-term project to develop an effective treatment process for mine water, it was soon recognized that the removal of soluble ferrous iron was the most difficult technical aspect of mine water treatment. The Bureau’s research on acid mine water treatment is now essentially completed and in the course of developing engineering costs for the limestone process for treating acid mine water and for optimizing and simplifying the process, the concept of catalytic oxidation of acid mine water was tested. This report gives the data and the results of exploratory work conducted in the laboratory.
Mine waters which contain iron and/or acid in excess of statutory standards must be treated before discharge to surface streams. The common method of treatment involves removal of total acidity by lime or limestone neutralization, aeration, and separation of settled solids.
The total acidity in mine waters comprises the free acid (usually sulfuric), and the mineral acidity generated by hydrolysis of metal salts, mainly of iron and aluminum. During the neutralization process, until an equilibrium is established, ferrous hydroxide forms and upon oxidation to ferric hydroxide yields a hydrogen ion. Therefore, each equivalent of ferrous hydroxide formed yields an equivalent of acidity. Consequently, the neutralization of total acidity is not instantaneous, especially when considerable ferrous iron is present. Usually, excess alkalinity must be supplied to the mine water during treatment to achieve a sufficiently high pH to maintain a low (less than 10 ppm) ferrous ion concentration in solution, and to neutralize the hydrogen ion concentration generated by the oxidation of ferrous hydroxide to ferric hydroxide.
The lime neutralization end point of a mine water that is continually changing in composition and flow rate cannot be adequately maintained by a simple pH control limit. This alkalinity leads to increased reagent costs with possible deleterious effects, since a high pH water can be as harmful as an inadequately treated water. The uncertainty inherent to adequate neutralization can be partly compensated for only by overdesigning the aeration equipment, and closely monitoring the alkalinity requirements of the mine water feed. With limestone treatment, the use of excess limestone to insure complete neutralization is not critical, since limestone costs are considerably less and an excess of limestone cannot produce an excessively high pH in the treated water. However, aeration requirements are increased and extensive equipment is required if considerable ferrous iron is present in the mine water.
Stoichiometric neutralization can be achieved with pH control if the mine water acidity is confined to free acidity and hydrolysis of ferric and aluminum sulfates. To achieve this, ferrous iron must be converted to the ferric iron form in the raw mine water. This would greatly simplify the neutralization process with attendant advantages of precise control and reduced treatment costs.
Activated carbon accelerates the rate of oxidation of ferrous iron in sulfuric acid solutions. Highly acid mine waters are very dilute solutions of sulfuric acid and acid salts. This paper presents the results of preliminary tests using an activated carbon to catalytically oxidize ferrous iron in acid mine water.
Acknowledgments
The cooperation of George S. Tobias and Ronald S. Joyce of the Technical Staff of the Pittsburgh Activated Carbon Division of Calgon Corporation in supplying test materials and in freely discussing catalytic oxidation is gratefully acknowledged.
Experimental Procedure
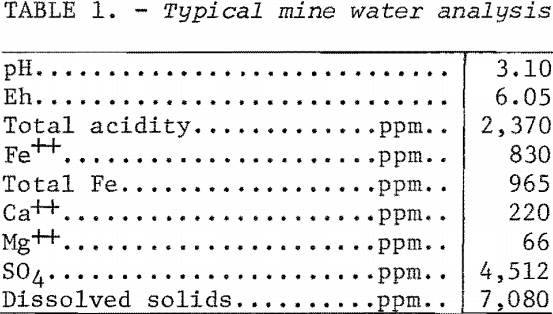
The carbon used throughout the tests was a commercial type coal-base activated carbon sized 12 by 40 mesh. Mine water containing 613 to 945 ppm Fe++, 810 to 960 ppm total Fe, 2,100 to 2,400 ppm total acidity, and pH ranging from 2.70 to 3.15 was used for the tests. A typical analysis of the mine water is shown in table 1.
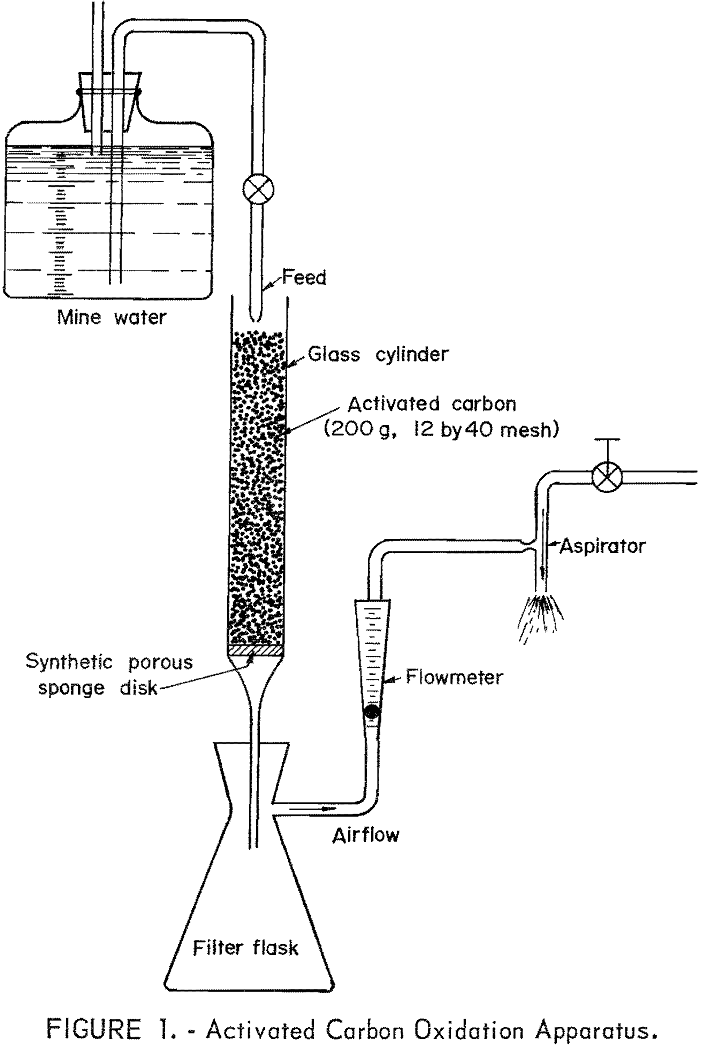
The experimental apparatus consisted of a glass cylinder with a fritted disk connected to a filtering flask. The disk supported the granular carbon and allowed passage of air and water. A side tube in the filtering flask was connected to an aspirator. The same batch of activated carbon (200 grams, 12 by 40 mesh) was used in all the tests. Two different glass cylinders were used, one in which the carbon column was 6.5 cm in diameter and 12.5 cm in depth; the other (latter tests) in which the carbon column was 3.8 cm in diameter and 42 cm in depth. The longer cylinder was used to increase flow through time. A synthetic porous sponge supported the granular carbon in the cylinder and prevented the loss of carbon particles. A sketch of the activated carbon oxidation apparatus is shown in figure 1.
Air was aspirated through the carbon column before the mine water was added, and continuously throughout the test until most of the water had passed through the carbon, The mine water volumes flowing through the carbon column ranged from 175 to 1,070 cu cm per batch test; air rates ranged from 0 to 3,500 cu cm per min.
Prior to testing, the batches of raw mine water were analyzed for pH, ferrous iron, and total iron. Identical analyses were made immediately on the effluent from the activated carbon column. All tests were conducted at room temperature.
A total of 65 separate runs, consisting of about 25 liters of raw mine water, were made in the same carbon column over a period of about 2 weeks.
Test Results
The pH of a suspension of the activated carbon in distilled water was about 6.6. With successive additions of the acid mine water (pH 2.70 to 3.15), the pH of the effluent decreased steadily and appeared to level off at a pH of 2.3 to 2.5 when ferrous iron oxidation was virtually complete. The raw mine water was practically colorless, but the effluent from the carbon column was a deep amber color due to the presence of a hydroxylated ferric sulfate.
The ferrous iron content (635 to 920 ppm) of the raw mine water was reduced to about 10 ppm, with a flow through time in the carbon column of less than 1 minute. By contrast, aeration of an acid mine water in the absence of an oxidizing agent, except for bacteria which may have been present, reduced the ferrous iron concentration from 261 to about 10 ppm in 168 hours. While a direct comparison cannot be made between these results, they do indicate the enormous increase in the ferrous iron oxidation rate that can be achieved with activated carbon.
Results of the tests are summarized in table 2. These preliminary results indicate that activated carbon catalyzed the air oxidation of ferrous iron in a ferruginous acid mine water. The initial tests also show that the activated carbon must be preconditioned to an acidic state for the catalytic reaction to take place. The low ferrous iron content in the effluent, beginning with run 26, was consistently maintained to the end of the experiment. It was likely that the activated carbon was adequately conditioned at this time. For these tests the total iron (practically all in the ferric form) of the effluent represents about 65 percent of the total iron in the feed.


After passing 25 liters of a ferruginous mine water through the carbon column, there is no appreciable loss in catalytic efficiency and no visible signs of surface fouling or solids deposition on the surface of the carbon particles; although a portion of the iron was adsorbed by the carbon. It is possible that only an insignificant amount of iron would be held by the carbon in a continuous flow operation.
Air requirements for oxidation of the ferrous iron might be minimal. Run 60, conducted without aspirated air through the carbon column, produced equally effective ferrous iron removal.
