Table of Contents
Solvent extraction of a waste solution from the phosphating of automobile bodies led to the following conclusions:
Separate zinc, nickel, and phosphate products, essentially free of each others were produced. Zinc was 99 percent extracted by di-2-ethylhexyl phosphoric acid in kerosene, and nickel was 99 percent extracted by dinonyl naphthalene sulfonic acid in butyl ether. Extraction efficiency is higher if zinc is extracted first. The zinc extraction was more efficient than the nickel extraction. Essentially all the phosphate remained in the aqueous raffinate. Stripping each organic phase with dilute sulfuric acid recovered the metal and regenerated the organic phase for recycling. About one-third of the sodium content of the waste reported in the nickel concentrate, and the balance stayed with the phosphate. Zinc and nickel were readily stripped from the organic phases but would probably require concentration to obtain useful products.
Modifications of this solvent extraction procedure might be useful in treating other waste solutions containing nickel or zinc.
The phosphate coating of metals is widely used in industry, especially in the manufacture of steel products, for several purposes: To provide a base to which paint or enamel will strongly adhere, for corrosion protection, for lubrication in die-forming operations, and to provide a porous surface that will absorb and retain lubricating oil. The phosphating solution consists essentially of phosphoric acid, a primary phosphate of iron, zinc, or manganese, and oxidizing agents such as nitrites, nitrates, chlorates, and peroxides. The phosphate coating reaction is based on the insolubility in water and solubility in acids of most metal phosphates. When steel is contacted with the phosphating solution, iron is dissolved, hydrogen is evolved, and the primary metal phosphates are converted to insoluble secondary and tertiary phosphates that deposit on and strongly bond to the metal surface. As hydrogen gas bubbles would slow the reaction, oxidizing agents are added to convert the evolved hydrogen to water. A typical overall reaction follows:
3 Me(H2PO4)2 + Fe(steel) + O → Me3(PO4)2 + FeHPO4 + H2O + 3 H3PO4
(where Me is iron, zinc, or manganese, and the underlined products are bonded to the metal surface).
The original metal surface is irregular, electrically conductive, and susceptible to corrosion; the coated surface is relatively smooth, nonconductive, and corrosion resistant. Commercial phosphating solutions are usually proprietary and contain other chemicals in addition to those mentioned. In addition, other metals may be dissolved from the metal undergoing treatment.
The wastes from such phosphating operations, which consist of spent phosphating solutions and an insoluble sludge, are often discharged to streams or ground waters. The steady increase in discharge of phosphate wastes into streams in the Missouri River Basin has been reported in two Bureau of Mines publications. Based on annual U.S. production of thin sheet steel and estimating that 20 percent of this steel is phosphated, it is estimated that several million gallons of phosphate wastes are dumped each year into streams and rivers in the United States. This large amount of waste contributes to stream pollution problems, and also represents large losses of nickel, zinc, and phosphates, together with lesser amounts of copper, manganese, and lead.
Phosphorus compounds, such as the phosphates in the wastes, are excellent nutrients for algae and other aquatic plants, whose growth causes oxygen depletion in streams and rivers. Even very small amounts of phosphates in rivers produce large amounts of algae. Certain metals in phosphate wastes also constitute pollution problems, as they are very toxic to fish and other organisms. These combined factors may cause the death of large numbers of fish, the sickness or death of livestock that drink from polluted streams, danger to municipal water supplies, and the closing of public swimming areas.
This problem has been neglected in the past, and the authors know of no other research being done on phosphate wastes from steel-coating operations. As a part of its program to conserve minerals and metals by recovering and recycling them through the economy, the Bureau of Mines is investigating this problem. The objective of the research reported herein was the technical evaluation of solvent extraction as a technique for the selective recovery of nickel and zinc from waste phosphate solutions used in phosphating automobile bodies in assembly plants. This work was preliminary to later research on solid phosphate wastes (sludges), which contain more kinds and larger quantities of metals and phosphates, and constitute a more difficult separation problem.
Materials and Procedures
This investigation consisted of two phases;
- Preliminary batch tests in separatory funnels to evaluate potential solvent extraction systems in which solvent, carrier, phase volume ratios, and other operating parameters were studied
- Continuous; countercurrent extractions to evaluate the data from preliminary tests and to obtain operating data for economic evaluation of the proposed technique.
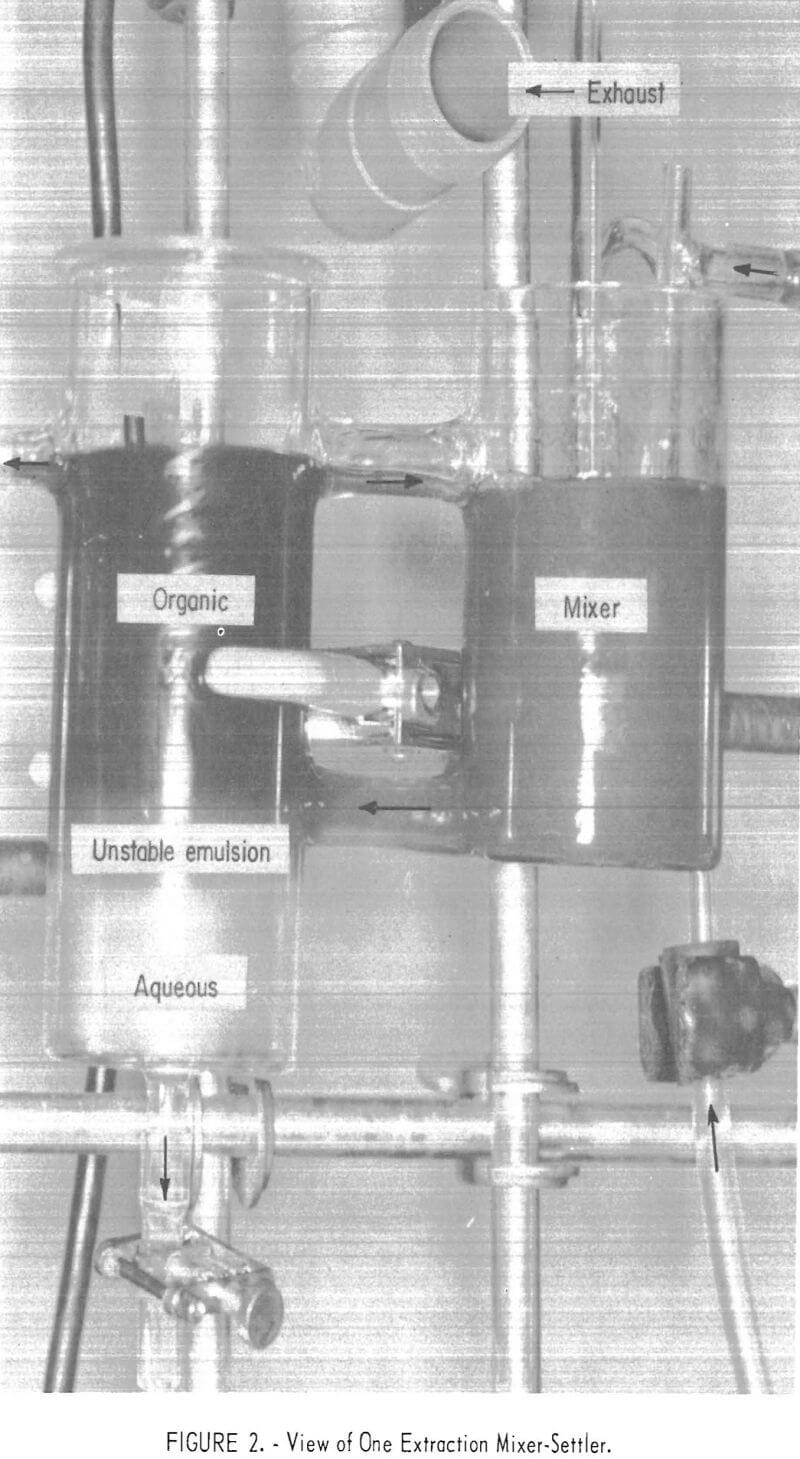
All tests were at room temperature, and in all tests zinc was extracted first, followed by the extraction of nickel from the zinc raffinate Partial analyses of the waste phosphate solution are given in table 1.
Chemistry of the Extractions
The preliminary extraction tests indicated that zinc could be selectively extracted from the aqueous phosphate solution by di-2-ethylhexyl phosphoric acid (EHPA) in kerosene. This extraction proceeds by cation exchange between the liquid phases. EHPA exists as a dimer, (RH)2, in kerosene. The generalized equation for the extraction is
![]()
As the equation shows, the progress of the extraction results in increased acidity of the aqueous phase, which was verified experimentally when the pH of the solution dropped from 3.5 to 2.5 during a single extraction. Stripping of the organic phase with dilute (15 volume-percent) H2SO4 recovers the zinc and regenerates the EHPA.
Other preliminary extraction tests showed that nickel could be selectively extracted from the phosphate solution by dinonyl naphthalene sulfonic acid (DNSA) in butyl ether. This extraction also proceeds by cation exchange. DNSA probably occurs as a polymer, (RH)x, in the solution. The equation for the extraction is
![]()
Stripping the organic phase with 15 volume-percent H2SO4 recovers the nickel and regenerates the DNSA. After the extraction of both zinc and nickel, the raffinate is largely phosphoric acid, sodium phosphate, and sodium nitrate.
Equipment and Procedure
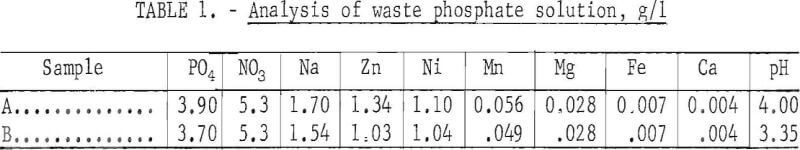
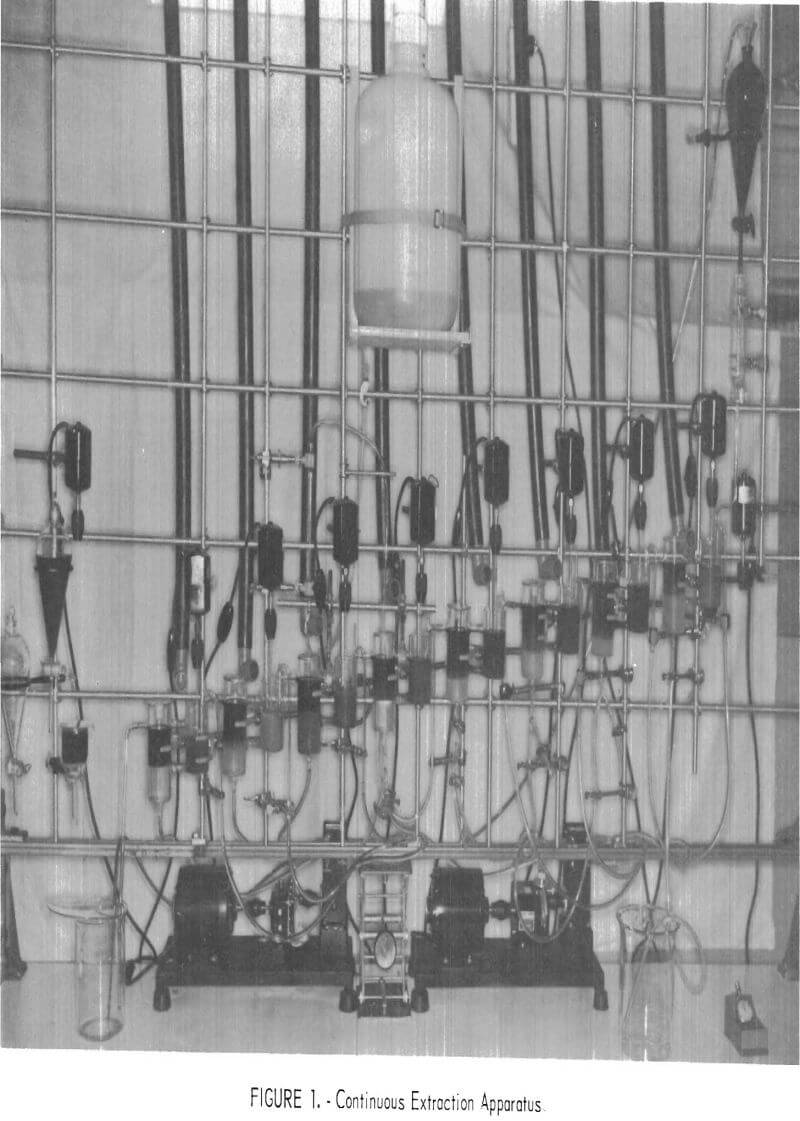
The continuous, countercurrent extraction tests were made, in the apparatus shown in figure 1. One of the Pyrex glass mixer-settler units (one extracttion stage) is shown in figure 2. After the two phases are contacted by stirring in the cell on the right, the phases flow to the left cell and separate. The process flowsheet is shown schematically in figure 3. In a typical test, the organic phase was introduced into the top, right-hand mixer-settler shown in figure 1 and allowed to flow by gravity through the desired number of extraction stages. The aqueous waste solution was introduced in one of the lower left mixer-settlers and was pumped upward from stage to stage for countercurrent extraction. The stripping cycle was similar except that sulfuric acid replaced the waste solution.
The volume of a mixer-settler (both cells in figure 2) was 500 ml at operating level. In the zinc circuit the organic phase was admitted at 8 ml per minute and the aqueous phase at 22 ml per minute; the volume ratio of organic phase to aqueous phase was, therefore, 1:2.75. In the nickel circuit the organic phase was admitted at 10 ml per minute and the aqueous phase at 12 ml per minute. The organic/aqueous ratio was 1:1.2. The liquid flow rates and volume ratios were not arbitrarily chosen; the flow rates were necessary for continuous operation in order to allow sufficient time for phase separations, and the volume ratios were based on extraction requirements as determined by single-stage experiments. Residence time in the mixer-settlers was longer than in the separatory funnel tests.
Reagents
The solvent for zinc, a 20-volume-percent solution of EHPA in commercial grade kerosene, was prepared from commercial grade EHPA obtained from Union Carbide Corporation. Prior to use, the solution was washed with dilute phosphoric acid (pH 2.5) to remove an unidentified material that caused turbidity in the aqueous phase during countercurrent experiments.
The solvent for nickel was prepared from commercial grade reagents obtained from R. T. Vanderbilt Company, Inc., and King Organic Chemicals, Inc. The DNSA samples as received were 36 percent and 41 percent DNSA in heptane. Twenty volume-percent of heptane solution in practical grade butyl ether was used to extract nickel.
Experimental Results
Investigation of Solvent- Extraction Variables
This investigation consisted of batch tests made in separatory funneIs.
pH of Aqueous Phase
The pH of the waste phosphate solutions from automobile assembly plants varied from 3.4 to 3.5. Because both phosphate and sodium ions were present in the solution, phosphoric acid and sodium hydroxide were used to adjust the pH of the solution to the desired values. Precipitation precluded the use of pH values above 4.5. One hundred milliliters of each phase were used for both zinc and nickel extractions, and to insure equilibration, 20 minutes contact time was used in all tests.
The distributions or zinc and nickel between solvent and aqueous phases as a function of the pH of the aqueous phase are shown in figure 4. The distribution coefficient E°a is directly proportional to the efficiency of the extraction and is defined as
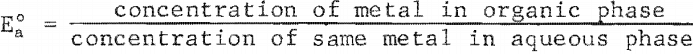
As the solvents used for zinc and nickel are very highly selective, product purity requirements are negligible in this particular case in deciding which metal should be extracted first. Figure 4 shows that zinc is extracted more efficiently as the pH value of the aqueous phase increases. At pH values of 2.5 or less, nickel is extracted more efficiently than zinc. Therefore, it is more efficient to extract the zinc first, at higher pH values, and then to extract the nickel at the lower pH values resulting from the zinc extraction. The pH value of the raffinate after the zinc extraction was approximately 2.5.
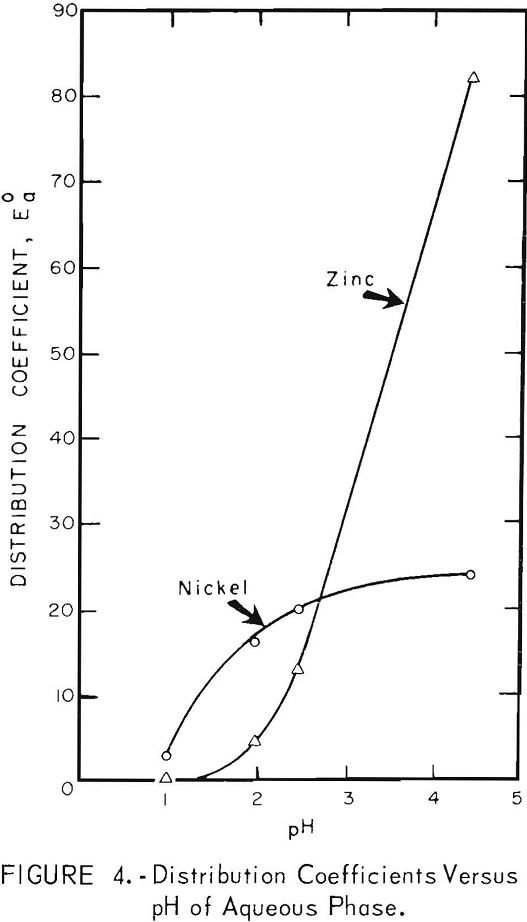
Contact Time
Preliminary tests showed that both zinc and nickel were extracted rapidly. One hundred milliliters of each phase were shaken together mechanically in a separatory funnel. The distribution of zinc and nickel between solvent and aqueous phases at various contact times are shown in figure 5. The contact times were the times that the two phases were shaken together; the slight additional time required for separation of phases was neglected. The results show that equilibrium was approached in 1 or 2 minutes in the nickel extraction, and in approximately 5 minutes for the zinc extraction.
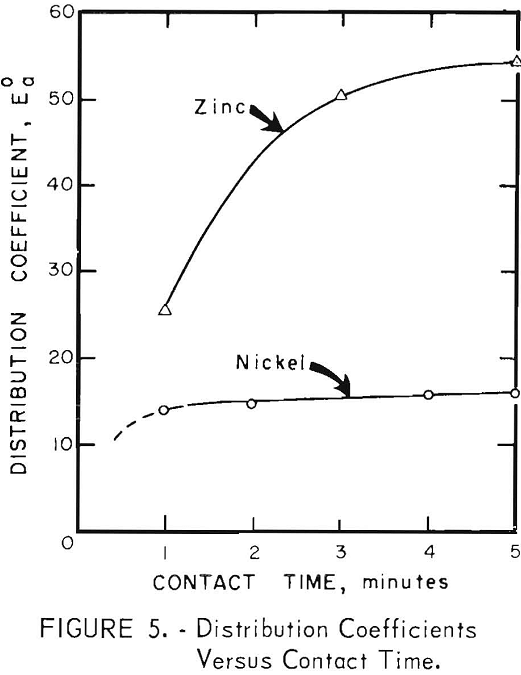
Organic/Aqueous Volume Ratio
The effect of varying the phase volume ratio is shown in figure 6. All tests were made by mechanical shaking for 20 minutes in a 500-ml separatory funnel. The results show that extraction efficiencies increase as the organic/aqueous ratio increases, and that for a given phase ratio the extraction of zinc is more efficient than that of nickel.
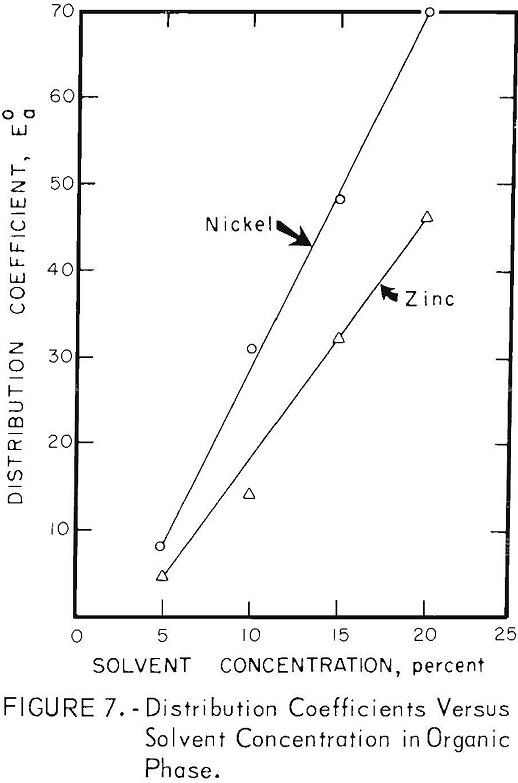
Solvent Concentration
The results of varying the concentrations of EHPA and DNSA in their organic carriers are shown in figure 7. Although extraction efficiencies increased with increasing solvent concentrations, increased time required for phase separation, due to emulsion formation, sets a practical limit of about 8 percent DNSA in butyl ether-heptane. Emulsion formation was not a problem in the zinc extraction; the 20-percent solution of EHPA in kerosene, used in most tests, was a compromise between extraction efficiency and solvent loss.
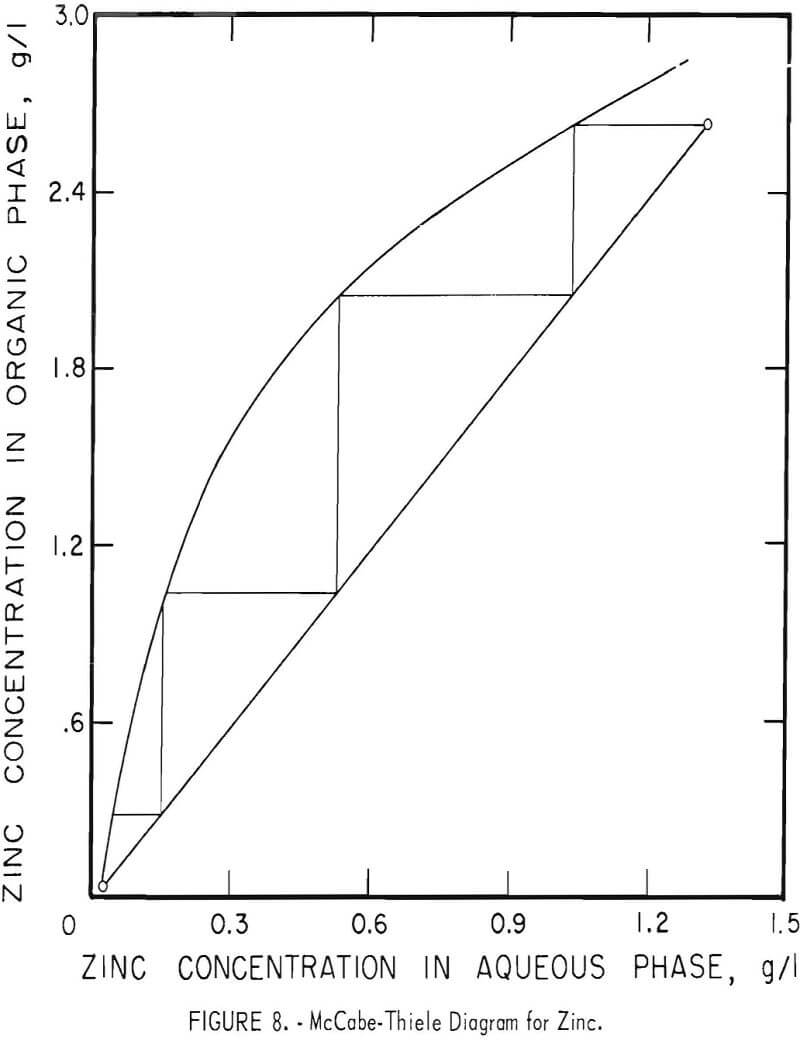
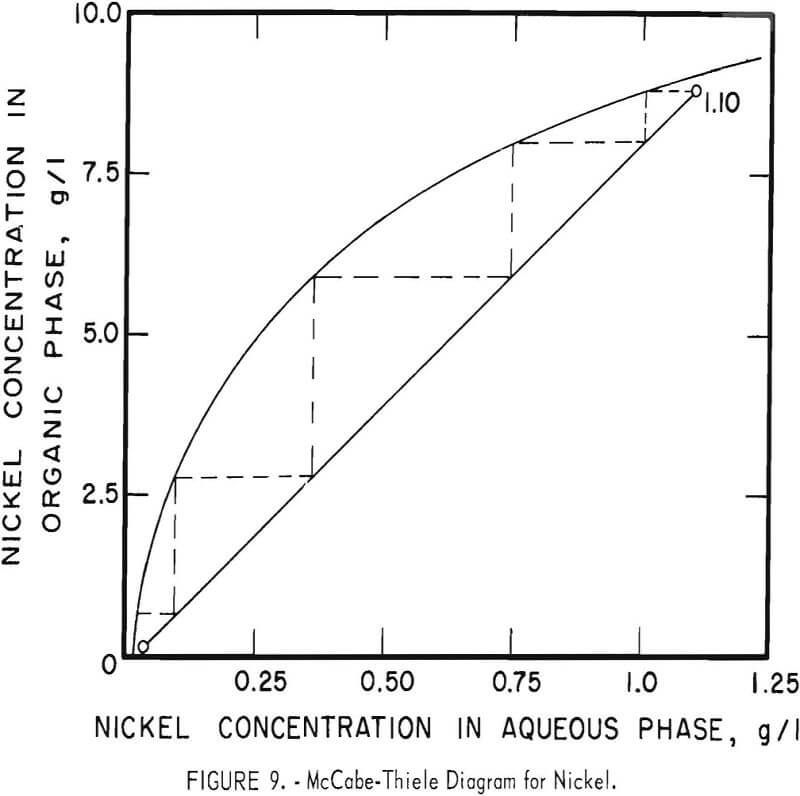
Theoretical Extraction Stages Required
The theoretical number of stages required for extracting zinc and nickel were determined for sample A by shaking 100-ml portions of feed (or of the zinc raffinate for nickel) with varying amounts of the organic extractant in a separatory funnel. Metal concentrations were determined in both phases and the results were used to construct the McCabe-Thiele diagrams shown in figures 8 and 9. These diagrams show that three or four stages would extract the zinc almost completely and that four or five stages would suffice for nickel.
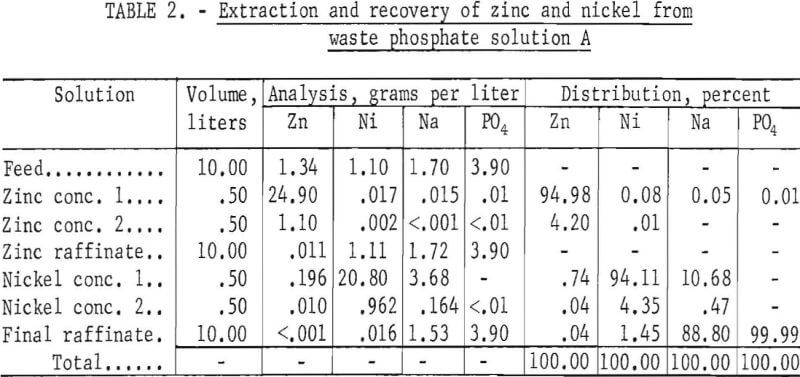
Continuous, Countercurrent Extraction and Recovery of Zinc and Nickel
Solution A
In an 8-hour countercurrent test in the apparatus shown in figures 1 and 2, zinc was extracted in three stages and nickel in four stages. Stripping was two stages each for zinc and nickel. Detailed results are given in table 2. Nearly 100 percent of the zinc and over 98 percent of the nickel were extracted from the waste phosphate solution. The zinc concentrate contained very little nickel, sodium, or phosphate. The raffinate from the zinc circuit, which was the feed to the nickel circuit, contained less than 1 per¬cent of the total zinc and practically all of the nickel, sodium, and phosphate
The final raffinate was essentially free of zinc, but contained 1.45 percent of the nickel, which indicated the need for another nickel extraction stage. Sodium was found in both the nickel concentrate and the final raffinate.
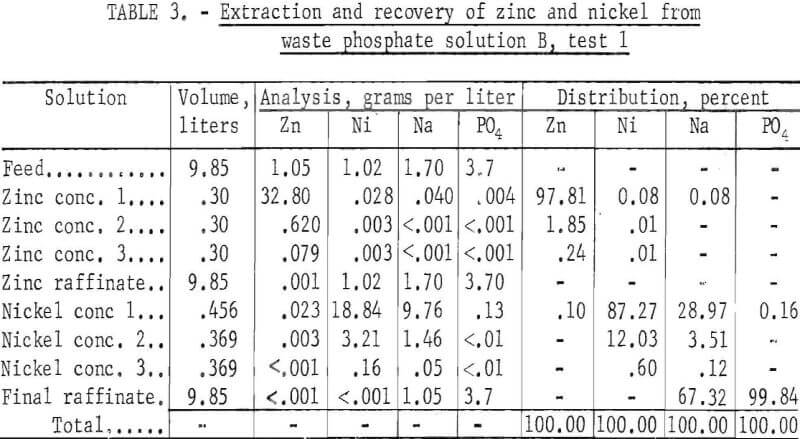
Solution B, Test 1
In this 8-hour test, three stages were used in the zinc circuit, five in the nickel circuit, and three in each of the stripping circuits. Results are given in table 3. Essentially all of the zinc and nickel were extracted from the waste phosphate solution, leaving behind essentially all of the phosphate. The sodium again split between the nickel concentrate and the final raffinate.
Solution B, Test 2
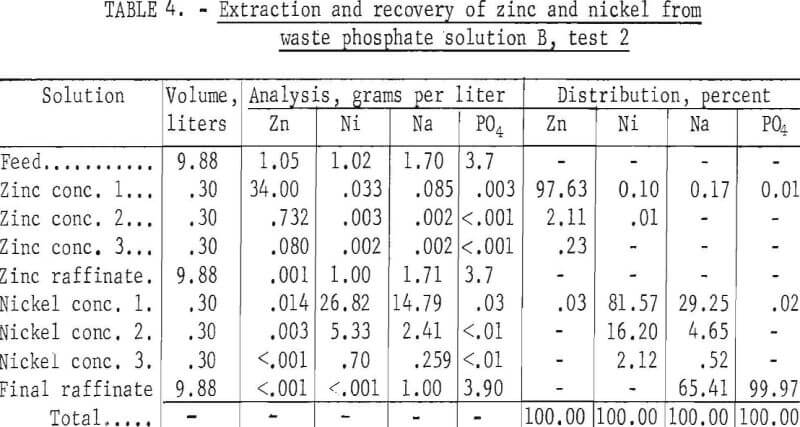
This test, a duplicate of test 1 on solution B, was made to verify the reproducibility of the results. Three stages were used in the zinc circuit, five in the nickel circuit, and three in each of the stripping circuits. The results, given in table 4, are essentially the same as for test 1: The zinc concentrate was practically free of nickel, sodium, and phosphate, the nickel concentrate was practically free of zinc and phosphate but contained about one-third of the sodium, and the final raffinate contained essentially all of the phosphate, none of the zinc and nickel, and about two-thirds of the sodium
Solvent Loss
Solvent losses, including those due to evaporation, solution, entrainment, and spillage, depend on equipment design and operating parameters, including organic/aqueous volume ratios, contact time, completeness of phase separation, and other variables. Therefore, solvent loss values calculated from bench- scale experiments should be regarded as rough approximations.
Evaporation losses were determined on stirred, isolated cells containing both phases, but no phase separation was made. Solution losses were determined after phase separation.
A 20-volume-percent solution of EHPA in kerosene showed evaporation losses as follows:
First 30 minutes……………………………………………..0.021 g/cm²
Next 60 minutes……………………………………………….012 g/cm²
Do……………………………………………………………………….Nil
Solution loss of this reagent was found to be 0.010 gram per 100 ml of solvent per hour of contact time in countercurrent operation. Both losses of this reagent are negligible.
Evaporation losses of DNSA in butyl ether are more serious because they are continuous, A 20-volume-percent solution of DNSA in butyl ether showed an evaporation loss of 0.021 g/cm² for the first 30 minutes, and a constant loss of 0.0065 g/cm² for succeeding 30-minute periods.
Solution losses of this reagent were found to be 0.064 gram per 100 ml of solvent of the 20-percent solution per hour of contact time in countercurrent extraction. Both evaporation and solution losses were due primarily to butyl ether and not DNSA.
Based on the above ether losses, the consumption of butyl ether per pound of nickel recovered in an average extraction experiment was 0.377 lb. At a cost of $0.36 per lb for butyl ether, this represents a reagent cost of $0.136 per lb of nickel.
Recovery of Phosphate and Nitrate from Final Raffinate
The relatively low unit value of the phosphate and nitrate in the final raffinate preclude a complex flowsheet for their separate and individual recovery. However, these products do have value and also constitute a serious pollution problem if discharged in quantity to streams. Therefore, their recovery was sought as a mixed fertilizer material that could be marketed locally to avoid transportation costs.
Simple chemical reaction with ammonia, ammonium hydroxide, or potassium hydroxide formed a mixed fertilizer material. This could be sold as a solution or the solid could be recovered for packaging. An alternate method involved reaction with the stoichiometric amount of sodium hydroxide to form the trisodium salt of the phosphate, which was recovered by precipitation with ethyl alcohol. The nitrate was then recovered as the ammonium or potassium salt. This alternate approach would be more costly, but with alcohol recovery by distillation, it might be desirable in some market areas.
