Table of Contents
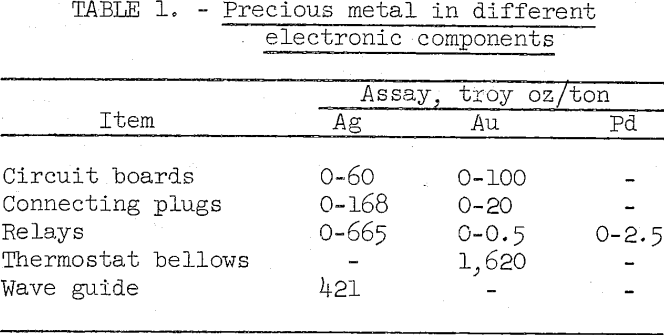
Gold, silver, and platinum group metals are widely used in electronic and electrical components to provide long-term reliability, Construction of military equipment consumes the largest proportion of the precious metals used in the electronic and electrical industry. Due to obsolescence and damage, military electronics are presently being scrapped at the rate of about 15,000 tons per year. This scrap averages approximately 100 troy ounces of silver, 5 troy ounces of gold, 1 troy ounce of palladium, and lesser amounts of other precious metals per ton.
The high level of development and use of military electronic hardware that soon becomes obsolete assures an expanding supply of scrap electronic components from military sources.
Because of its highly variable and complex nature, military electronic scrap is practically impossible to sample and analyze for metal content. This fact coupled with the inflexibility of presently used scrap-processing methods, limits disposal to a relatively small part of the scrap that is now generated and has been accumulated by military reclamation operations. The Bureau of Mines is currently investigating alternative methods for efficient, low-cost recovery of the precious and base metals in diverse mixtures of military electronic scrap.
Leaching techniques were tried initially for processing the electronic scrap. Nitric acid dissolved copper and silver but left a residue from which the gold could not be separated. Cyanide leaching proved ineffective for dissolving heavy silver and gold plate on copper. The large amounts of soluble copper and aluminum in typical scrap made leaching complicated and costly.
After preliminary studies revealed that simple leaching techniques were not applicable, the investigation was shifted to devising- a smelting method capable of recovering the precious metals and copper. The following is a brief account of the progress made toward solving the problem. Some of the remaining, unsolved problems are also discussed.
Nature of Military Electronic Scrap
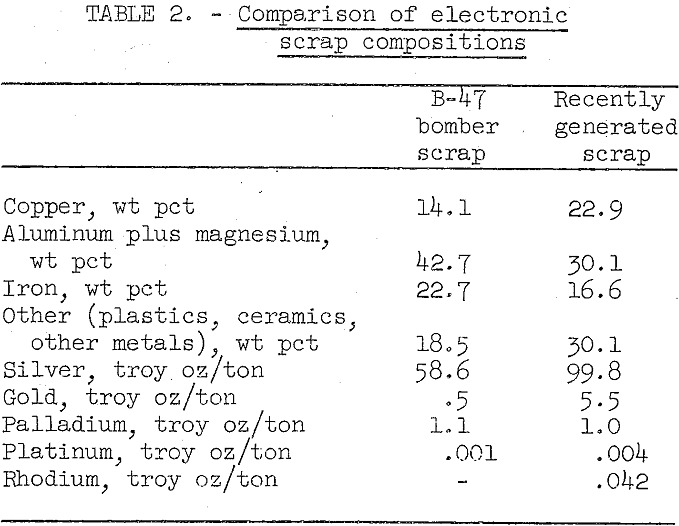
The nature and composition of military scrap is highly variable. While scrap from obsolete B-47 bombers comprises wired-vacuum tube-type components with relatively low precious metal content, the electronic components of later gear are composed of printed and miniaturized circuits of more complex nature and high precious metal content. In the more modern electronic components, gold and platinum group metals have replaced part of the silver used in older type electronics. With miniaturization, use of plastics has increased, and the amount of aluminum and iron encasing the components has decreased significantly.
Precious metal assays of some typical electronic components are shown in table 1.
Table 2 shows a comparison between the composition of B-47 bomber electronic components and the composition of a 400-pound lot of more recently generated scrap.
Smelting Studies
Typical military electronic scrap can be economically processed together with copper sulfide concentrate, in a conventional copper smelter to recover the copper and precious metals which constitute the principal values. Unfortunately, this simple solution to the problem is not practical, because such scrap cannot be sampled and assayed to define its composition, and hence to determine the smelter charges and payments. The only apparent alternative is to devise techniques for batchwise electric furnace smelting of small tonnages of complex scrap.
Military electronic scrap contains relatively large amounts of iron and aluminum which require oxidation and slagging to produce high-grade metal bullion containing the copper and the precious metals. Because slagging a large part of the furnace feed was certain to create difficulties, the feasibility of rejecting clean iron and aluminum prior to smelting was examined. Magnetic, hand-sorting, and sink-float techniques were tried on samples of electronic scrap shredded in hammer mills. None of these methods was effective for significantly lowering the iron and aluminum content of the scrap without high loss of precious metals and copper.
Smelting High Aluminum Electronic Scrap
Several small and subsequently one larger scale smelting test were made on samples of typical high aluminum and iron content scrap. In the larger test, 458 pounds of scrap were smelted at 1,500° C in an electric arc furnace. A small amount of silica was added just prior to j pouring the melt and the dross layer skimmed out of the furnace. The results are summarized in table 3.
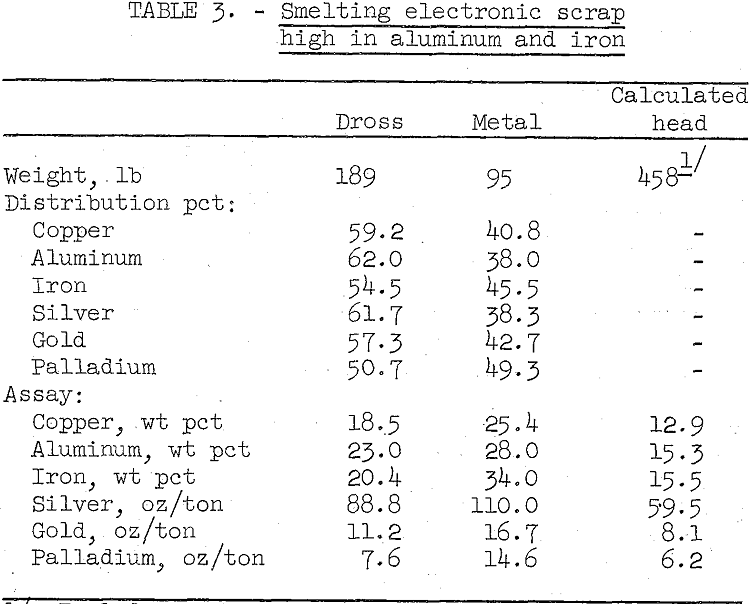
As shown by the data in table 3, the extremely viscous dross which assayed 23.0 percent aluminum and 20.4 percent iron retained 59 percent of the copper and over 50 percent of the precious metals, chiefly as small particles of metal. The metal bullion produced was contaminated with considerable iron and aluminum that was not oxidized. Although an easily handled clean dross and high quality bullion were not produced, the two products can be accurately sampled and presumably marketed to a custom copper smelter.
Smelting with additions of sufficient silica to flux and slag all the aluminum and iron also was unsuccessful. The slag formed was extremely viscous even at 1,500° C, and its relatively large volume retained a large part of the copper and precious metal values.
Smelting Low Aluminum Scrap
Low aluminum scrap that contains 33 percent iron was successfully smelted’using the same conditions as tried on high aluminum scrap. The results are summarized in table 4.
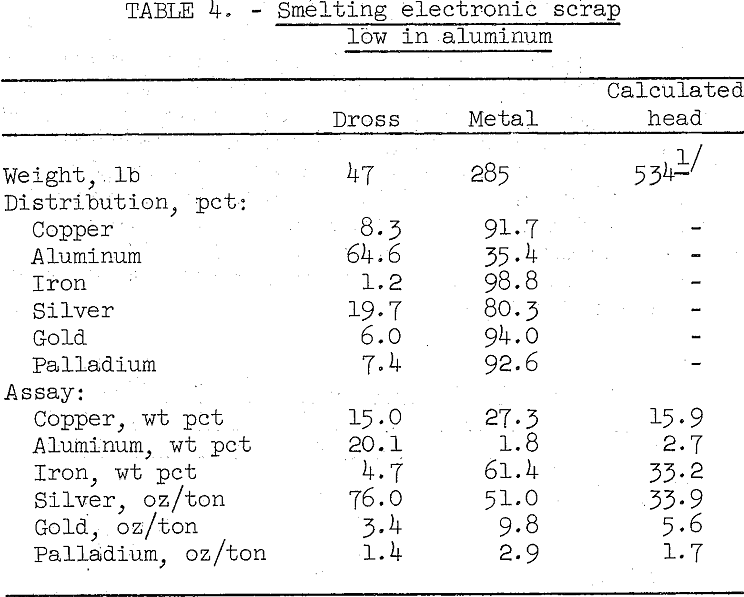
The relatively small amount of dross was easy to skim from the furnace. However, this product still contained too much copper and precious metals to be discarded, and the metal bullion produced is contaminated with considerable iron. Both products can be sampled, accurately assayed, and sold to a custom primary copper smelter.
Oxidation Smelting and Electrorefining of Low-Aluminum Scrap
Another possible option for processing electronic scrap is to smelt suitable low-aluminum scrap to produce a copper bullion low in iron for subsequent electrorefining to recover high-purity copper and a saleable precious metal rich sludge.
Oxidation Smelting
In practice, the initial reduction smelting of scrap can be followed immediately by oxidation smelting and slagging of the iron and aluminum to produce a high quality copper bullion. However, in the laboratory these operations were done separately to enable evaluating the merit of completing only the initial reduction smelting.
The crude bullion produced from low aluminum scrap and described in table 4 was used for the oxidation smelting test. After remelting the bullion in a gas-fired pot furnace, the temperature was raised to about 1,500° C, and air was blown through the charge for 5 hours. Approximately 0.3 pound of silica per pound of bullion was added to the melt during the blowing operation. Slag was skimmed off, the melt was poled, and the metal was poured into cast iron molds. Over 95 percent of the copper and precious metals were recovered in the bullion, and over 99.5 percent of the iron and aluminum were slagged off. Table 5 shows the assay of the purified bullion.
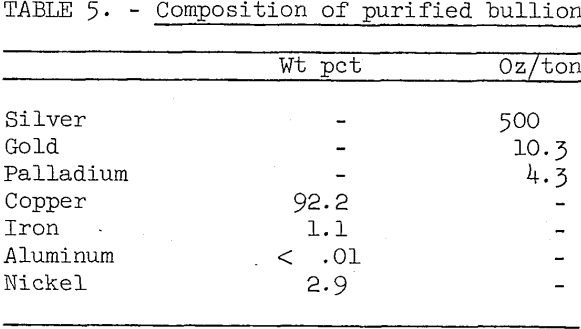
Aluminum and iron rejection was relatively good however, the iron concentration in the copper is somewhat higher, and the nickel and precious metal content are much higher than in normal blister copper. The concentrations of other metals are low. If sold to a conventional copper smelter, such bullion would be so diluted with normal blister copper as to provoke no problems in anode refining or electrorefining.
Electrolytic Refining
The copper bullion made by oxidation smelting is considerably higher in nickel, iron, gold, and silver than the anode copper produced by normal copper smelter practice. Some difficulty was anticipated in preparing copper meeting the specifications for electrolytic grade. To examine this problem, electrolytic tests were made simulating standard copper refinery practice. The copper sulfate electrolyte contained 45 grams copper, 200 grams free sulfuric acid, 4 milligrams-glue, and 40 milligrams sodium chloride per liter. Electrolyte temperature was 50° C and the current density was 15 amp per square foot of cathode area. Table 6 shows assays of the anode used and the cathode and anode slimes produced in a typical test.
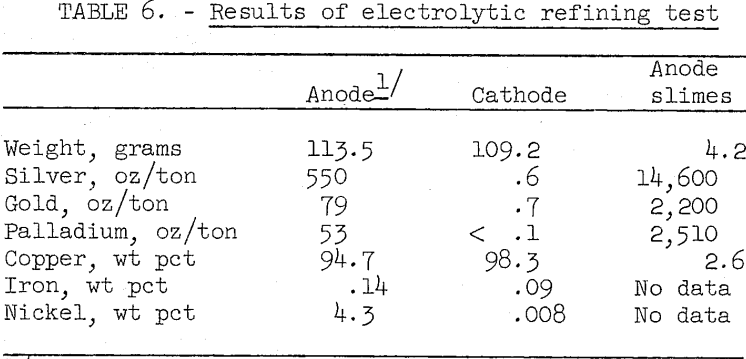
The impurities in the cathode copper are approximately 10 times greater than generally encountered in industrial practice. Due to the high nickel content in the anode copper, an auxiliary nickel recovery unit would be required. This operation is a standard auxiliary procedure in many refineries and should not present any serious problems. A second electrolysis to further refine the copper and recover the remaining precious metals may be advisable. Research is continuing to attempt to lower the impurity content in the first cathode.
No experimental work has been done on the treatment of the anode slimes for the recovery of precious metals. The slimes are similar to those produced:in standard electrolytic copper refinery operations and.should respond to normal treatment procedures for the recovery of the various precious metals.
Plans For Future Work
The research goal to devise techniques sufficiently flexible for efficient processing of all types of complex, high-value military electronic scrap has not, as yet, been achieved. The principal unsolved problem is how to selectively remove and preferably recover the relatively large amount of aluminum present in much of the scrap. Smelting aluminum-rich scrap is difficult and costly; furthermore, smelting loses the aluminum in valueless dross and slag. Incineration of plastics, either prior to or concurrently with smelting, creates a pollution problem and also may represent a possible loss of precious metals by volatilization. Studies are needed to evaluate various methods for controlling pollution, as well as recovering values which may be carried in the fumes.
Several methods are being studied and evaluated for recovering aluminum from scrap prior to smelting. These include (1) dismantling the scrap to remove the aluminum boxes and shields that encase the electronic components, (2) caustic leaching to selectively dissolve the aluminum, (3) sweating aluminum from the electronic scrap, and (4) separating aluminum from shredded and incinerated scrap, using molten salt to float the lower density aluminum from the other metals.
Summary
Because complex military electronic scrap cannot be accurately sampled and assayed, such scrap is not marketable for simple and economic processing by copper smelters. Attempts to separate and recover the valuable precious and base metals by leach techniques were not successful. Smelting tests indicated that low aluminum scrap can be processed to produce slag and crude bullion saleable to a copper smelter. Alternatively, the copper bullion can be electrorefined to recover the bulk of the copper and precious metals. High aluminum scrap can be smelted only with difficulty to produce a relatively large amount of dross and a crude bullion. These products can be sampled, assayed, and presumably further processed at a custom processing copper smelter. Magnetic, sink-float, and hand-sorting techniques were unsuccessful in’ rejecting aluminum’and iron from shredded scrap to prepare an enriched, easily smelted product.
