Table of Contents
Cement copper production in the United States has increased during the past few years, principally because more submill-grade material from open-pit operations is being leached in waste dumps. Since 1960, the cement copper annual growth rate has been about twice the rate for primary copper. If this trend continues, annual production of copper from cement copper precipitates will be 550,000 tons by 1975.
In commercial cementation plants, shredded detined cans are the chief source of iron used as the copper precipitant. The cement copper produced contains from 60 to 90 percent copper. Oxygen is the principal impurity owing to partial oxidation of the moist, fine copper particles during drying. Copper precipitates also are contaminated with silica, unreacted iron, and extraneous debris associated with tin cans. Standard practice is to smelt this impure cement copper in the reverberatory furnace along with copper sulfide flotation concentrates. Because of the increasing production of cement copper and absence of excess capacity at existing smelters, alternative processing routes to present smelting practice should be considered.
The direct smelting and fire refining of cement copper in an electric furnace was investigated by the Bureau of Mines in the early sixties. This approach has not been commercially adopted. Other approaches now being practiced on a commercial scale are dissolution of some of the cement copper for electrowinning at Inspiration, dissolution of cement copper in aerated, hot sulfuric acid solution, followed by hydrogen reduction in an autoclave to produce a powder as practiced at Bagdad, and solvent extraction coupled with electrowinning in lieu of cementation as practiced at Bluebird. Because of growing markets and a premium price for copper powder, we elected to examine the preparation of powder as a route for disposing of cement copper. Electrolysis of sintered anodes to make conventional cathode copper was also examined.
Cement Copper Samples
Cement copper samples were obtained from six different operating plants. The samples all contained small amounts of coarse iron and foreign matter which were rejected by screening on a 10-mesh sieve. The minus 10-mesh fraction, which contained about 99 percent of the copper, was chemically analyzed. The composition of the principal components is given in table 1.
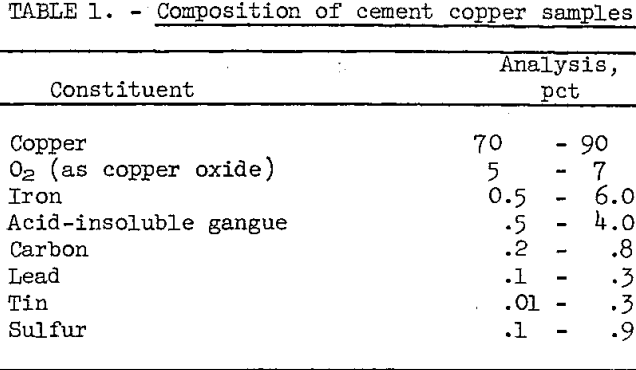
The calcium, cadmium, manganesium, manganese, nickel, and titanium contents of the six samples were less than 0.1 percent each. Oxygen, as copper oxide coating on the cement copper, was the principal impurity in all the samples. The other impurities remaining after screening were derived mainly from the iron precipitant. These impurities included unreacted iron, iron oxides, sand, glass, fabric, paper, and rubber.
Copper Powder Process
The term “powder,” as used in this report, implies particles having the required size, chemical composition, and physical characteristics for use in the powder metallurgy industry. Presently, commercial powders are produced by the hydrogen reduction of copper oxide or electrolytic precipitation of copper sponge. A method developed in the laboratory but not used commercially precipitates copper from solution with high-grade iron and processes the high-grade cement copper to powder.
The current investigation was concerned with upgrading impure commercially produced cement copper to produce a marketable copper powder. The process as developed comprised (1) screening to reject coarse iron and foreign material, (2) flotation to reject the insoluble material and other impurities, (3) chemical purification with acidulated copper sulfate solution to dissolve residual iron and aluminum, (4) hydrogen reduction and sintering to decrease the oxide content and consolidate the fine-sized copper, and (5) grinding the sinter cake to a powder.
Magnetic fractionation of the minus 10-mesh cement copper removed about 65 percent of the remaining iron and 5 percent of the copper. In commercial practice, the magnetic fraction probably could be recycled to copper cementation. As this was not practicable in the laboratory work, magnetic treatment was not included in the flowsheet.
Flotation of Cement Copper
Preliminary flotation tests indicated that a dixanthogen-type reagent (Minerec A), which served as both collector and frother, gave results superior to those obtained with other types of copper collectors. Use of supplemental frothers decreased the selectivity of the process, and use of depressants for the gangue material decreased the copper recovery.
Detailed tests then were made to define the effects of different flotation condition variables. Of the many factors evaluated, the reagent amount, froth depth, pulp temperature, and pH proved to have significant effects on the flotation of cement-copper stage-ground to pass 100-mesh.
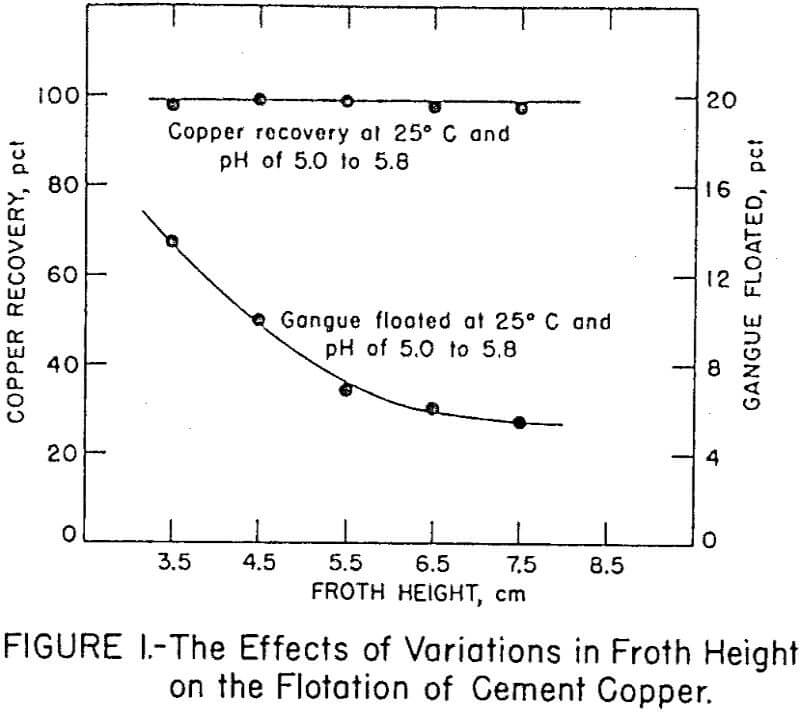
Stage additions of collector to both rougher and cleaner flotation steps proved essential for achieving maximum selectivity. Amounts of Minerec A required for maximum recovery in rougher flotation and two stages of cleaner flotation were 0.67 and 0.28 pound per ton of cement copper, respectively. In all the tests, the initial pulp density for flotation was 22 percent solids and the final pulp density was about 4 percent solids. The influence of froth column height on copper recovery and acid-insoluble gangue flotation is shown in Figure 1. The figure shows that copper recovery did not vary significantly with different froth depths,but the flotation of gangue decreased with increased froth depth. Apparently the greater froth depth enabled better differential drainage of entrapped pulp from the bubbles.
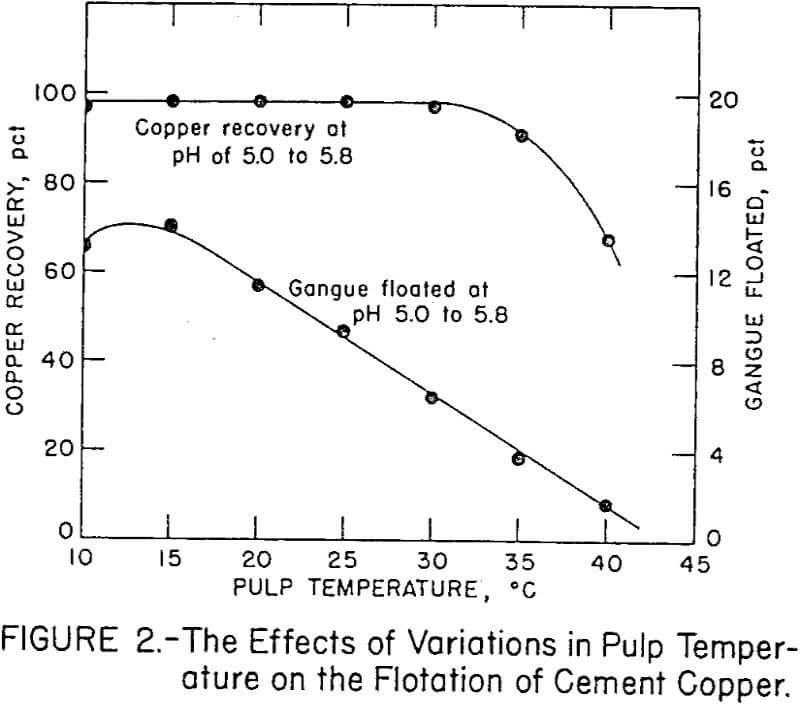
Temperature variations in the flotation pulp were maintained within ½° C by employing a constant-temperature circulaton with a water-jacketed flotation cell. Figure 2 shows the effect of pulp temperature on copper and gangue flotation from the cement copper. Copper recovery decreased above a pulp temperature of 35° C. The optimum temperature for high copper and low gangue flotation was 30° C.
The natural pulp pH for the cement copper sample used in this series of flotation tests was 5.8. Lower pH’s were obtained by adding H2SO4 to the pulp and higher pH’s by adding CaO. The variations in pulp pH and corresponding recoveries are shown in table 2.
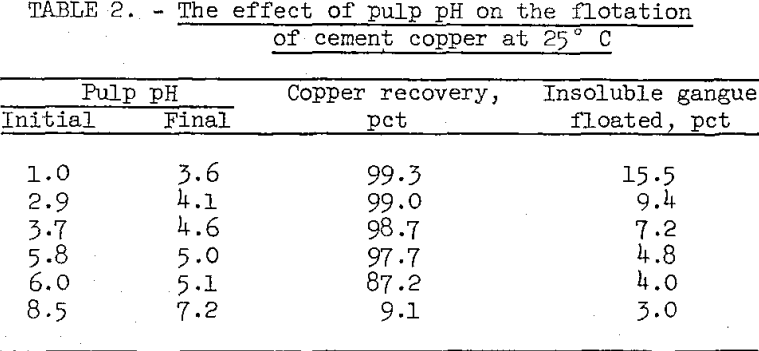
The tests showed that best results were obtained by floating in the natural pH range of the cement copper pulp (5.8 to 5.0). Copper recoveries could be improved slightly by reducing initial flotation pH to about 3, but flotation of insoluble gangue material also increased. Poor copper recoveries were obtained at pH’s higher than the natural pulp pH.
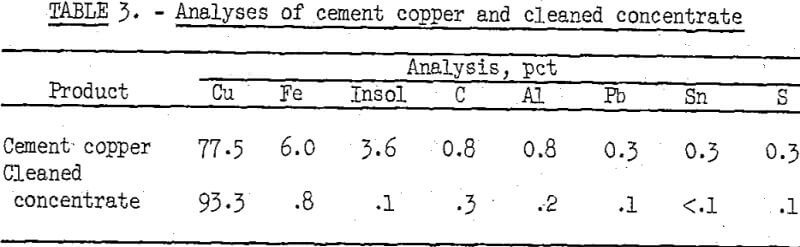
After the best conditions for effective flotation were determined, a sample of one of the lower grade cement copper samples was froth floated to prepare an upgraded concentrate for further processing. Overall copper recovery in the cleaned concentrate was 97.3 percent. The chemical analyses of the cement copper and the cleaned concentrate are given in table 3.
Chemical Purification of Flotation Concentrate
The beneficiated copper, which still contained significant amounts of iron and aluminum, was further purified by digestion in dilute sulfuric acid or acidulated copper sulfate solution. The digestion variables investigated were (1) solution composition, (2) digestion time, and (3) digestion temperature. No agitation was employed during digestion because the aeration induced by agitation resulted in excessive dissolution of copper.
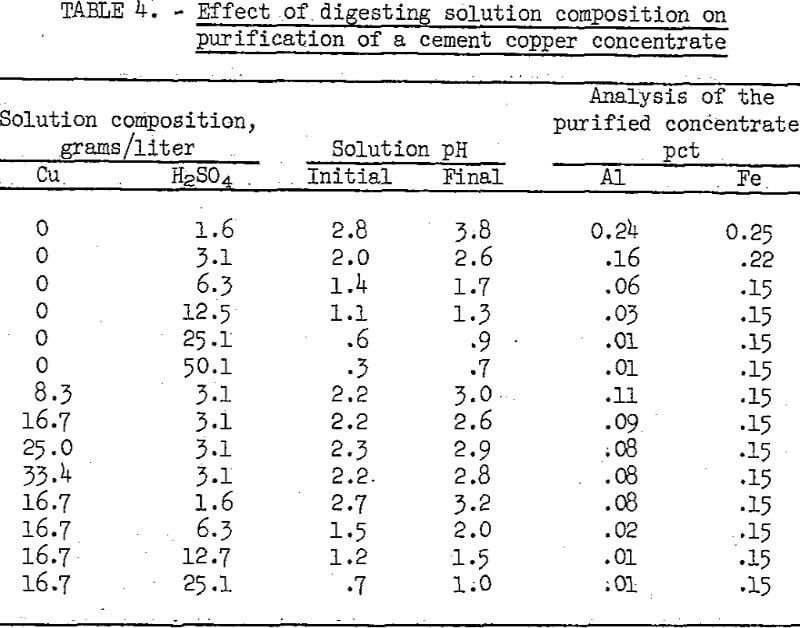
The effects of variations in the digestion solution composition are shown in table 4. For these tests the digestion time and temperature used were 60 minutes and 50° C, and the digestion pulp density was 10 percent solids. The test data show that equivalent purification was achieved with a solution containing either 25 grams H2SO4 per liter, or 16.7 grams Cu. and 12.7 grams H2SO4 per liter. When no copper was present minimum aluminum content was achieved with a pH of 0.9 in the acid solution, whereas when copper ions were present minimum aluminum was achieved at a pH of 1.5. Also, the minimum iron content was obtained at a higher pH with copper ions in the digesting solution.
Variations in time and temperature were studied concurrently with differences in digesting solutions. The results indicated that as the temperature increased the time required for digesting with either solution decreased. For example, with the 25 grams H2SO4 per liter solution, comparable purification was obtained by leaching for 5 hours at 30° C, 1 hour at 50° C, or 30 minutes at 80° C.
As much as 2 percent of the copper dissolved at maximum digestion time, temperature, and acidity of the solution. However, the leach solution can be recycled until impurity buildup necessitates replacement at which time the copper can be recovered by iron cementation. Satisfactory purification with dissolution of only 1.5 percent of the copper was obtained by digestion for 1 hour at 50° C using a solution containing 16.7 grams Cu and 12.7 grams H2SO4 per liter. When a sample of the beneficiated cement copper, reported in table 3, was purified by leaching under these conditions, the end product contained, in percent, 93.3 Cu, 0.1 Fe, 0.1 acid-insoluble gangue, 0.3 C, 0.1 S, 0.1 Pb, 0.05 Al, and 0.05 Sn.
Reduction, Sintering and Grinding
Definite specifications for the many different uses of copper powders are nonexistent. In general, the evaluation of a copper powder requires processing a test lot of the powder to the desired end product. If satisfactory, the chemical and physical properties of the test lot powder are determined and these become the specifications.
Making a complete evaluation of copper powders by end use was not feasible within the scope of the investigation. Instead, the chemical and physical properties of the copper powders prepared from cement copper were compared with the similar properties of commercial powders. The properties of three different types of commercially produced copper powders are given in table 5.
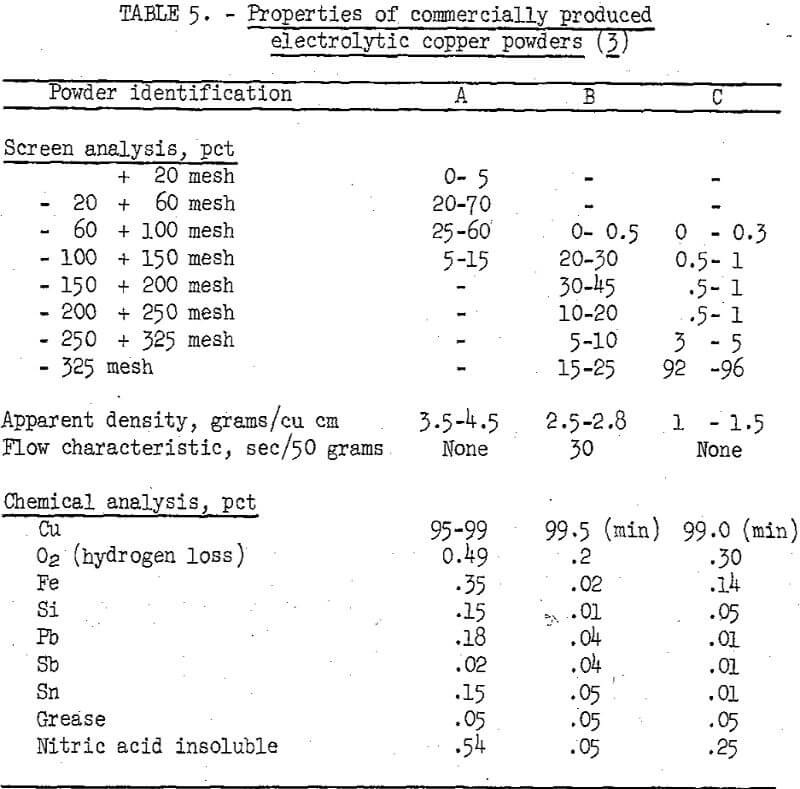
The measurable physical properties are apparent density, flow characteristics, particle size, and particle size distribution. These properties were defined using as a guide methods established by the Metal Powder Association. The particle size and particle size distribution of the various powders were determined using Tyler standard screens and a mechanical shaking device. The flow characteristics of the ground powder were determined with a Standard Hall Flowmeter and evaluated by the number of seconds required for 50 grams of sample to pass through a calibrated orifice. The flowmeter was equipped with a calibrated 25-cubic-centimeter receiving cup that was used for determining apparent densities.
Chemically purified cement copper flotation concentrates were reduced and sintered in a tube-type furnace while a flow of hydrogen was maintained over the charge. Sinter cakes were produced at various temperatures and ground to a minus-60-mesh powder in a swing hammer mill; the physical properties of the powders were measured. Ninety minutes sintering time was used for each temperature. The sintering temperatures and test results are listed in table 6.
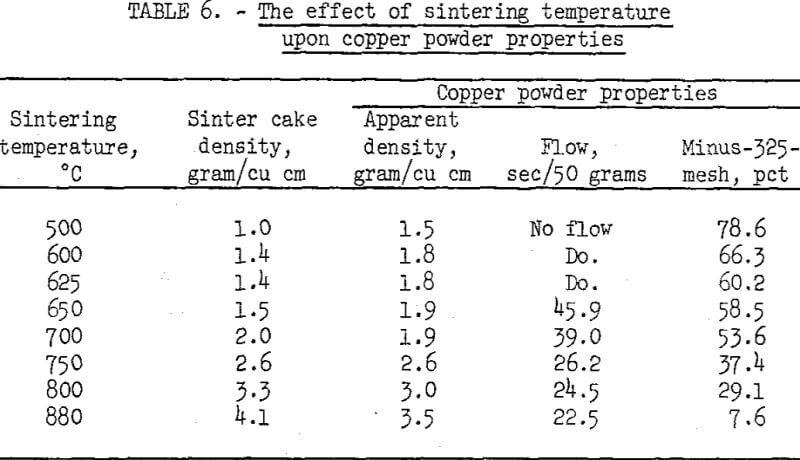
The data show that the physical properties of the copper powders are dependent upon the sintering temperature. As the temperature was increased from 500° to 880° C, the apparent density increased from 1.5 to 3.5 grams per cubic centimeter. Below 650° C, the powders would not flow, and above 650° C, the flow rate increased with increased temperature. All the copper powders produced had properties comparable to those of commercial powders.
The effect of sintering time on the properties of copper powders also was studied using a 750° C temperature. The results obtained indicated that as sintering time was increased from 15 to 120 minutes, the apparent density increased from 2.1 to 2.7 grams per cubic centimeter and the flow rate from 35.1 to 28.6 seconds for 50-gram batches. Like sintering temperature, the length of the sinter period provides a flexible method for controlling the physical properties of the copper powder.
After evaluating the effect of sinter temperature and time, a large test lot of the beneficiated and chemically purified cement copper (table 3) was reduced and sintered at 700° C for 1 hour, and then ground in a swing hammer mill to a minus-60-mesh powder. The physical properties and chemical analysis of the end product are given in table 7.
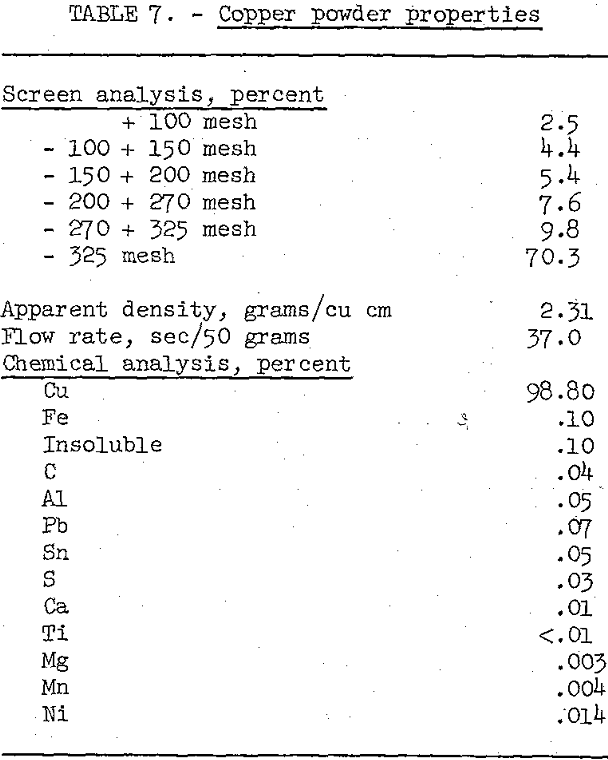
Comparison of the chemical analyses of the copper product before and after reductive sintering reveals that sintering increased the grade from 93.3 to 98.8 percent copper and decreased the sulfur and carbon to less than 0.04 percent. Both the physical and chemical properties of this copper powder are within the limits of commercially produced powders and, therefore, should be acceptable for industrial applications.
Electrorefining of Sintered Anodes
Although marketable-grade metal powder can be produced from cement copper precipitates, the amount of copper in cement precipitate greatly exceeds the present demand for purified copper powder. The electrorefining of sintered anodes of purified cement copper to conventional cathode copper was therefore studied. The initial steps comprise the same flotation, chemical purification, and sintering procedures used for the production of powder. Published copper refining data were used as a guide for selecting electrolytic conditions.
The anodes were made by reducing and sintering chemically purified cement copper concentrate in a hydrogen atmosphere at 800° C for 90 minutes. Use of the higher sintering temperature, in contrast to the 700° C used for the preparation of copper powder, was for the purpose of preparing anodes that would not slough during electrolysis. The anodes were well sintered and firm, and had a density of 3.3 grams per cubic centimeter. The chemical analysis of the anodes is given in table 8.
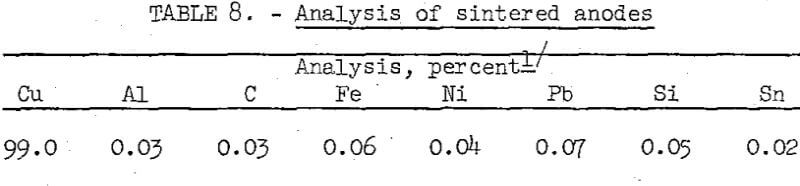
The electrorefining cell was a modified 1,500-ml beaker capped with a Lucite lid. Two 16-gauge cathode starting sheets, 1.5 inches wide by 3 inches high, were placed opposite the 1.25-inch-wide by 2.5-inch high by ½-inch-thick sintered anode. Anode to cathode spacing was 1.5 inches. The anode was immersed in 800 ml of electrolyte to a depth of 1.6 inches, while cathode immersion was 2.1 inches. Operational limitations of the small laboratory electrolytic cell made it necessary to enclose the anode in a fabric bag to prevent sludge particles from contaminating the cathode.
A series of tests were made to determine the effect of copper concentration in the electrolyte on current density, current efficiency, and power consumption. Sintered anodes were electrorefined at 55° C using electrolyte containing 15, 30, and 40 grams Cu and 210 grams H2SO4 per liter. Anode depletion was carried to 100 percent for comparison of test parameters. Cathode current densities ranged from 15 to 35 amperes per square foot at 0.14 to 0.40 volt. For each electrolyte concentration, a limiting current density of 35 amperes per square foot was reached at 0.32 volt. Current efficiencies were all 99 percent or greater. Power consumption was directly proportional to the current density and ranged from 108 kwhr per ton of copper at 15 amperes per square foot to 246 kwhr per ton at 35 amperes per square foot. Operating data were similar at all electrolytic concentrations, but a slight improvement in current density was obtained with 30 grams Cu per liter.
Further tests were made refining successive anodes to determine the effect of impurity accumulation in the electrolyte on cell operation. The electrolyte composition was 30 grams Cu per liter and 210 grams H2SO4 per liter, and the temperature was 55° C. At a current density of 30 amperes per square foot, the cell voltage was 0.24 volt. Seven anodes were electrorefined in succession, and a total of 274 hours of cell operation was completed before current efficiency decreased significantly. During this period, the current efficiencies averaged 99 percent, and the power consumption averaged 185 kwhr per ton of copper. These results compare favorably with industrial practice. The average amount of anode sludge formed was 0.8 percent by weight of the refined copper. The cathode copper produced contained 99.99 percent Cu and impurity contamination of the electrolyte was minimal.
Although the small-scale tests showed that the sintered anodes produced were structurally sound and could be refined successfully, more work needs to be done on larger anodes to determine if commercial-scale anodes would crumble during electrorefining. Another approach presently being compared with sintered anode electrorefining comprises reduction smelting of the upgraded cement copper and casting into anodes for electrorefining. Preliminary tests on a flotation concentrate containing 93 percent Cu, with oxygen as the principal impurity, resulted in anode-grade metal containing 98.4 percent Cu. Fire refining of this product instead of electrorefining also will be examined because of the absence of precious metals in cement copper.
Cost Evaluation
Cost estimates were made for processing cement copper by the copper powder and sintered anode electrorefining processes described. Because smaller plants probably would be more interested in producing copper powder than installing expensive electrorefining facilities, the powder process was evaluated for a plant having a capacity for producing 10 tons of copper powder per day. For comparative purposes, plants producing 25 tons per day of powder or cathode copper were evaluated. Operating costs were estimated on the basis of a 350-day rated capacity operating year. The plants were depreciated over a life of 12.5 years. For the 10-ton-per-day copper powder plant, costing $765,000, the unit production cost is 5 cents per pound of copper. For a 25-ton-per-day copper powder plant, costing $1,325,000, the production cost is 3 cents per pound of copper. It is difficult to extrapolate the sintered anode electrorefining process to a commercial scale based on the operation of the small-scale electrolytic tests. However, assuming the sintered anodes, would hold together and the anode size and spacing would be the same as in conventional electrorefining, a 25-ton-per-day plant for converting cement copper to refined cathodes would cost $2,300,000 and have a production cost of 5 cents per pound of copper.
Conclusions
The laboratory investigation demonstrated that a commercially produced cement copper containing, in percent, 77.5 Cu, 6.0 Fe, and 3.6 insoluble material could be upgraded by flotation, chemical digestion, and hydrogen reduction to a product containing 98.8, 0.10, and 0.10 percent of copper, iron, and insoluble material, respectively. The powders prepared by grinding this product had properties comparable to those of commercial copper powders. Variations in sintering time and sintering temperature were demonstrated to be effective means of controlling powder properties to meet different consumer specifications.
Alternatively, sintered anodes of upgraded cement copper could be electrorefined in the laboratory at a current density of 30 amperes per square foot to produce refined copper of 99.99 percent grade.
Based upon the results of the small-scale tests, the copper powder process appears to be a feasible alternative to conventional smelting as a means of converting cement copper to a marketable product. More work remains to be done before the feasibility of electrorefining sintered or cast anodes can be assessed.