Table of Contents
A series of tests were run on fresh Becker drill samples from a drill hole in Kennecott’s Chino “J” dump, near Silver City, New Mexico, to obtain data on the leaching chemistry of a relatively impermeable sulfide copper waste dump. Vertical profiles were made of the moisture and soluble-salt content; the content of Fe+³, Fe+², Cu+², and H+ in the interstitial solutions; and the content (both total and non-water soluble) of iron, copper, and sulfur in the residues. Conclusions were drawn from these data concerning the leaching chemistry and hydrological nature of this dump.
This study was similar to a previous study conducted on other, more permeable leach dumps at Chino. Briefly, the conclusions of the first study are as follows:
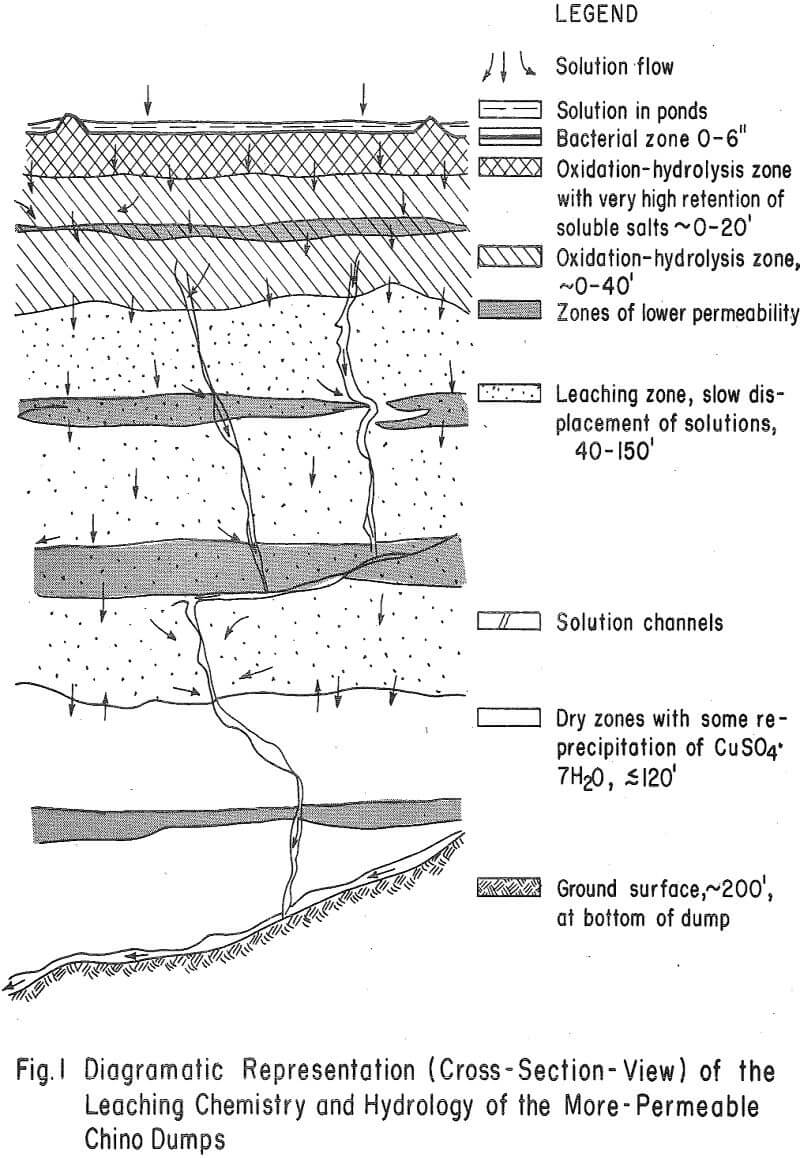
- Solutions are transported through major portions of more-or-less permeable dumps at variable and rather slow flow rates according to the permeability of the strata. Some short-circuiting of solutions through the dump, however, is indicated.
- The oxidation and hydrolysis of iron-bearing solutions occurs in the upper 40 feet of these dumps. This phenomenon appears to be a result of bacterial, air, and ground-water oxidation of a near-surface zone of retained iron and copper salts. The cyclic addition of leach fluids and the manner in which they are added (sprinkling or ponding) appear to be important factors in the accumulation of soluble salts in this iron oxidation-hydrolysis zone.
- A reasonable degree of copper dissolution occurs within the wetted zones of these dumps as a result of contact with the oxidized leach solutions. Some evidence, however, indicates there to be rather inefficient removal of solubilized copper from some areas of the dumps by the leach solutions.
Figure 1 diagramatically shows the hydrology and chemistry of permeable-dump leaching, as determined by this early study.
Experimental Procedure
Eight-foot interval, pneumatic, Becker drill samples were analyzed for their moisture contents, soluble-salt contents, the chemical compositions of the interstitial solutions, and the chemical compositions of the rinsed and unrinsed solids within a week after they were collected at Chino.
The following procedure was used to obtain these data: (1) Each of the 8-foot-interval samples was crushed to -3 mesh and split into
two 300-g fractions. (2) Moisture contents were determined by weighing, drying and reweighing one of these 300-g fractions. Copper, iron, and sulfur analyses were run on these residues. (3) The other 300-g sample was rinsed on a Buchener filter with 50 ml of solution adjusted to a pH of 2.80 with H2SO4 just before rinsing. In a separate rinse, the solids were further rinsed with 900 ml of water, dried, reweighed, and analyzed for iron, copper, and sulfur. The difference in the dried, rinsed weight of this sample, and the dried weight of the unrinsed sample was considered to be the weight of the soluble salts. The 50-ml rinse solutions were analyzed for copper, total iron, ferrous iron, and (pH).
Experimental Data
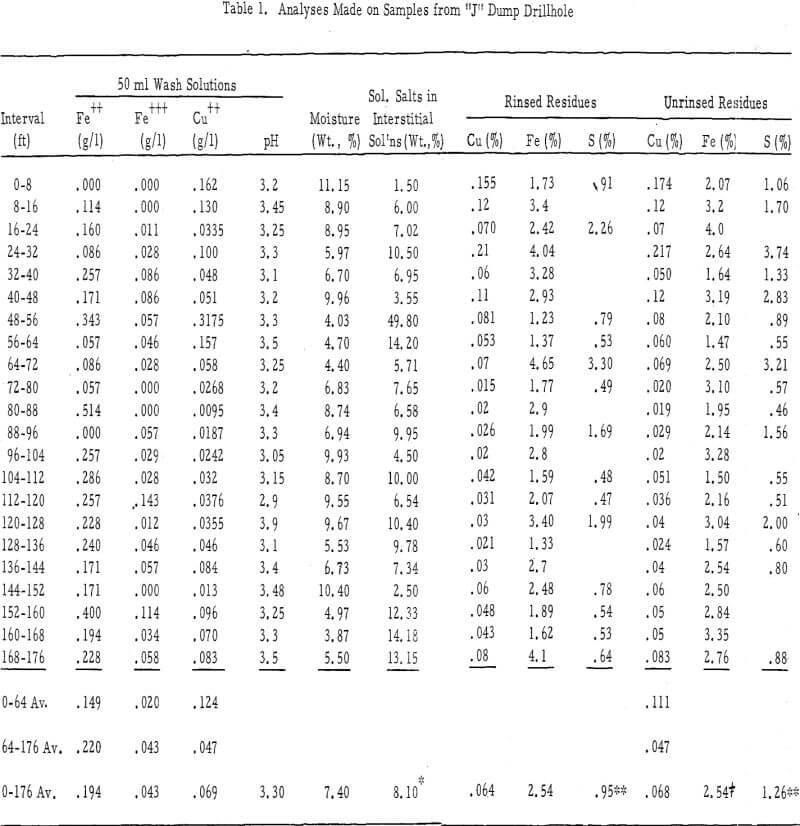
Test data (see table 1), tabulated in the order of their vertical profiles, show the content of Fe+³, Fe+², Cu+², and H in the interstitial solutions, moisture contents, the percentages of soluble salts in the interstitial solutions, and the iron, copper, and sulfur contents of the rinsed and unrinsed residues.
Discussion of Experimental Results
Moisture Content – The test data show the average moisture content of the upper 176 feet of the “J” dump to be 7.40 percent. Wide variances are evident, however, within vertical zones of the dump. There are several zones where the dump is almost dry (about 2.5 to
5 percent moisture) and several where the samples were either partially or completely saturated with solutions (8 to 12 percent moisture) and several where the samples were either partially or completely saturated with solutions (8 to 12 percent moisture).
The moisture content of the more permeable dumps, studied previously, averaged in the 8 to 9 percent range and varied within a relatively narrow range with dump depth.
Soluble Salts – There was very little soluble salt buildup in the interstitial solutions of the “J” dump. The average value, exclusive of the high value in the 48-56 foot zone, was 8.10 weight percent. Seven of the 22 samples, all of which came from dry zones, showed values of more than 10 percent. The upper 8 foot zone of this dump contained only 1. 5 percent dissolved solids in the interstitial solutions, whereas leach solutions coming onto the dump have averaged about 5.6 percent.
These findings are in sharp contrast to those obtained in the previous study on the more permeable leach dumps. The earlier data showed very large buildups of soluble salts within the upper zone of the dumps and relatively high and uniform concentrations through-out the remainder of their depth.
Chemical Composition of Interstitial Solutions – Observations on the distribution and amount of ferrous and ferric iron, copper, and H+ in the interstitial solutions are as follows:
- There was no buildup of ferric salts within the interstitial solutions of this dump, and in only one or two instances was there a significant amount of ferrous iron. The ferrous iron to ferric iron ratio was high in all interstitial-solution samples.
- The pH of the interstitial solutions was above 3.1 (average of 50-ml, 2.80-pH wash solutions equaled 3.30) throughout the 176 feet of dump tested. Undoubtedly, this lack of acid in the interstitial solutions was responsible for their very low ferric iron content. The pH values of the interstitial solutions in the more permeable Chino dump (average of the 50-ml, 3.0-pH rinse solutions were 2.94 and 2.86) were much lower than those in this dump.
- The copper content of interstitial solutions was low (the average 50-ml wash solution was 0.124 gpl) throughout the upper 64 feet of this dump and very low (the average was 0.047 gpl in the 50-ml wash solutions) throughout the remainder of its drilled depth. One exception to the above was the 48-foot to 56-foot zone where the copper content of the 50-ml rinse solution was 0.318 gpl. The average copper contents of 50-ml wash solutions from the more permeable Chino leach dumps were 0.129 gpl and 0. 478 gpl.
- The average ratios of Fe+³ :Fe+² :Cu+² were 0.16:1.20:1.00 for the first 64 feet, 0.92:4.70:1. 00 for the next 112 feet, and 0. 62:2. 80:1. 00 for the total 176 feet drilled. These ratios were 0. 45:0. 67:1. 00 and 0. 35:0. 13:1. 0 below the 32-foot depths for the interstitial solutions of the more permeable dumps.
- The average ferrous, ferric, copper, and acid concentrations (about 0.40 gpl Fe+², 0.08 gpl Fe+³, 0.14 gpl Cu+², and 3. 60 pH) of these interstitial solutions were very low compared to the average assays of the total combined dump effluent solutions that are obtained at Chino (approximately 1. 0 gpl Fe+², 1.3 gpl Fe+³, 1.0 gpl Cu+², and 2. 5 pH).
Composition of Rinsed and Unrinsed Residues – Rinsed and unrinsed residue samples were assayed for copper, total iron, and sulfur. These samples averaged 0. 068 percent total copper and 0. 064 percent non-water-soluble copper; 2.54 percent total iron and 2.54 percent non-water-soluble iron; and 1. 26 percent total sulfur and 0.95 percent non-water soluble sulfur.
The distribution of copper, iron, and sulfur within the dump was as follows:
Total copper was considerably higher in the upper 64 feet (average = 0.111) of the dump than in the 64- to 176-foot
zone (average = 0.047).
Sulfur assays, although rather inconsistent, showed some evidence of segregation at the top of the dump. The inconsistent nature of these assays, lack of copper at depth, and the lack of other evidence of iron oxidation and hydrolysis, would indicate that the anomalous amount of sulfur in the top of this dump is in the form of copper and iron sulfides and not as a basic iron sulfate product, formed as a result of oxidation and hydrolysis of iron solutions.
Interpretation of Data
The three areas in which these data may contribute to an under-standing of waste-dump leaching chemistry are: (1) ore-solution contact or the hydrological nature of the dump (2) iron oxidation and hydrolysis and (3) the dissolution and removal of copper from the dumps. Interpretations made on these data are diagramatically shown in figure 2.
Ore-Solution Contact – Solutions appear to be transported at relatively high flow rates through select zones or layers of the dump. Evidence of this is (1) the variable moisture profile of the dump, (2) the high ratio of Fe+² to Fe+³, Cu+², and H+ in the interstitial solutions of wet zones, (3) the low content of ferric iron, copper, and H+ in the interstitial solutions, (4) the small amount of retained soluble salts, and (5) the low rates of copper dissolution within both dry and wet strata. Apparently the more permeable layers of this dump act as channels for the ingress and egress of relatively fast-flowing leach liquors. There
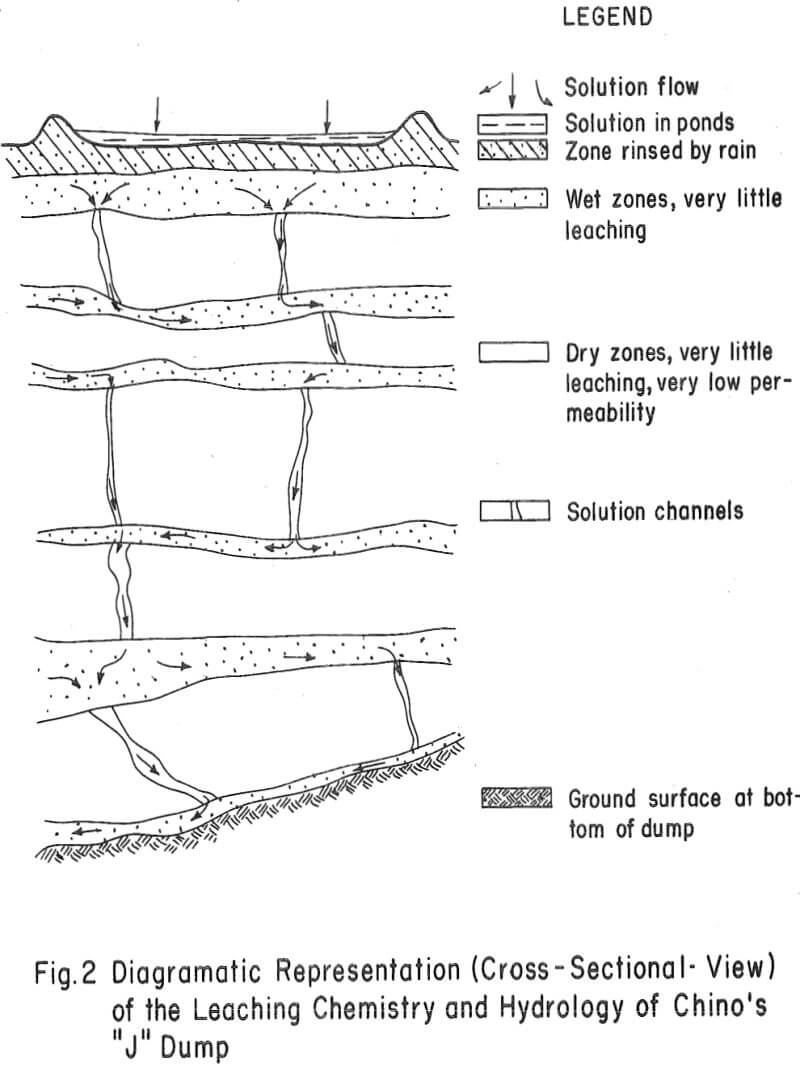
also appears to be but slight penetration of solutions or redeposition of soluble salts within the dry or impermeable zones.
Correlative gamma logging and tritrium tracer studies on this dump, by the U. S. Bureau of Mines, Kennecott Copper Corporation, and the New Mexico Bureau of Mines and Mineral Resources, have also borne out these observations.
Iron Oxidation and Hydrolysis – There is strong evidence that this dump does not have an iron sulfate oxidation and hydrolysis zone near the surface, as was found to be the case with more permeable Chino dumps. The data show the following: (1) There is no buildup of soluble salts in the upper layer of the dump. In fact, rain water appears to have rinsed the soluble salts from this zone to a level considerably below that of the leach liquors flowing onto the top of the dump. (2) There is no buildup of soluble ferrous iron, ferric iron, copper, or acid in the upper zone of the dump. (3) There is no evidence of ferric iron being formed in the interstitial solutions of the dump. These solutions consist mostly of ferrous iron with little or no acid and very little copper. (4) Residue assays do not support the idea that there has been a precipitation of an insoluble basic iron sulfate product in the upper portion of this dump as a result of ferrous iron oxidation and hydrolysis reactions.
This lack of oxidation and hydrolysis of the leach liquors suggests that bacteria are either not present within this dump, or if they are present, they are not active in converting ferrous iron to ferric iron and acid.
Dissolution and Removal of Copper from the Dumps – There appears to be very little dissolution and removal of copper from the Chino “J” dump. This observation is supported by the fact that (1) the interstitial solutions are very low in their copper content in both the wet and dry zones of the dump and (2) the stagnant nature of the dry zones.
The reasons for these low rates of copper dissolution and removal are: (1) there is very little, if any, ferric iron and acid available for dissolving the copper minerals, and (2) the penetration of solutions into the dry areas of the dump is poor, and hence there is little rinsing or flushing of mineral values from the dump. The fact that the lower two-thirds of this dump is very low in grade may also contribute to the poor extractions of copper from this dump.
In the previous study it was stated that several means are available for dissolving copper sulfides in waste dumps, i.e. dissolution with acid-ferric sulfate solutions, alternate wetting and drying cycles, and electrolytic reactions between minerals and neutral salt-acid reactions. The low rate of copper dissolution within the “J” dump indicates that the most important means of dissolving copper minerals in dump leaching is oxidation and dissolution of copper minerals with acid-ferric sulfate solutions. Very little leaching seems to occur when acid and/or ferric iron are absent from the interstitial solutions.
Conclusions
This study indicates the nature of leaching in the Chino “J” dump to be as follows:
- This dump is wetted in select horizontal zones and vertical channels by relatively fast-flowing ferrous sulfate solutions.
- There is no iron sulfate oxidation and hydrolysis zone within this dump. Hence, there is no means of generating acid and ferric sulfate within the leach liquors.
- Because of the short-circuiting of solutions through the dump and the lack of a means of generating acid-ferric sulfate, there is little or no leaching occurring within this dump.
These results are in sharp contrast to those obtained in a previous, similar study on some more permeable Chino dumps. In the earlier studies, solution flow was found to be slower and more uniform and to penetrate more of the dump; the influent ferrous sulfate solutions were oxidized and hydrolyzed to acid and ferric sulfate in the upper portions of the dump by a soluble-salt retention-oxidation mechanism and relatively good copper dissolution and removal from the dump was affected.
Acknowledgments
The authors sincerely express their appreciation to the New Mexico Bureau of Mines and Mineral Resources, a division of the New Mexico Institute of Mining and Technology, for permission to present this report of investigation. Appreciation is also extended to Mrs. Lynn Brandvold and a large number of undergraduate and graduate student assistants for their able help in analytical work and metallurgical testings; to Mr. William Arnold for drafting; and to the secretarial staff of the Bureau for typing and reproducing the manuscript. Especially, the authors express their gratitude to the management and staff of the Chino Mines Division, Kennecott Copper Corp. , Hurley, New Mexico for their cooperation and support throughout the project and for furnishing the samples for laboratory studies.
Leaching Tests on “J” Dump Core Samples
During emplacement of mine waste for dump leaching, compacted layers of low permeability are produced by heavy trucks and bulldozers that transport and spread the rock. Migration of fines and precipitation of salts after leaching starts form other impermeable layers. As part of a cooperative’ study concerned with improved leaching of compacted and cemented dumps, percolation leaching tests were made at our laboratory on drill core samples from a previously leached dump (“J” dump at the Kennecott operation near Santa Rita, N. Mex.) to determine what copper extraction could be expected if the dump were made permeable. This report presents the results of our mineralogic examination and leaching tests.
Descriptions of Samples
The “J” dump was systematically sampled by means of a Becker pneumatic drilling machine. Nine holes, from 130 feet to 207 feet deep, were drilled to bedrock from the top of the dump. Samples of the 3-inch-diameter drill cores were collected at 8-foot intervals.
The core splits received at Salt Lake City were essentially all minus-1 inch in size. A mineralogical study was made on an 8-foot interval (168- to 176-foot section) of a core sample (LDH-164) obtained from near the center of the drill hole array to identify the predominant copper and clay minerals. The sample contained, in percent, 0.22 total Cu, 3.1 Fe, 0.21 sulfide S, and 0.9 sulfate S. Examination with binocular and petrographic microscopes revealed that the section was composed of quartz, altered feldspar, sericite, clay, chlorite, magnetite, limonite, pyrite, chalcopyrite, covellite, chalcocite, and isolated grains of metallic copper. No discrete oxide copper minerals were found. The sulfate sulfur is in the form of water-soluble iron, magnesium, and manganese rather than any distinct mineralogical species. Virtually all of the sulfide minerals occur as small inclusions in the gangue material. Some chalcopyrite occurs as locked grains with the pyrite.
Differential thermal analysis was performed on the slime fraction of the sample to determine the clay minerals. The prepared clay sample, when heated, showed endothermic peaks at 100° to 200° C, representing loss of loosely held or absorbed water; at 325° C, representing impurities in the sample; at 550° to 610° C, corresponding to loss of (OH)-; and a small peak at 900° C, corresponding to disruption of the remaining structure. This curve is in close agreement with curves produced from illite samples. The chemical formula most commonly assigned to illite is (OH)4K2(Si6Al2)Al4O20 and the theoretical composition is, in percent, 45.2 SiO2, 38.5 Al2O3, 11.8 K2O, and 4.5 H2O.
Laboratory Percolation Leaching Tests
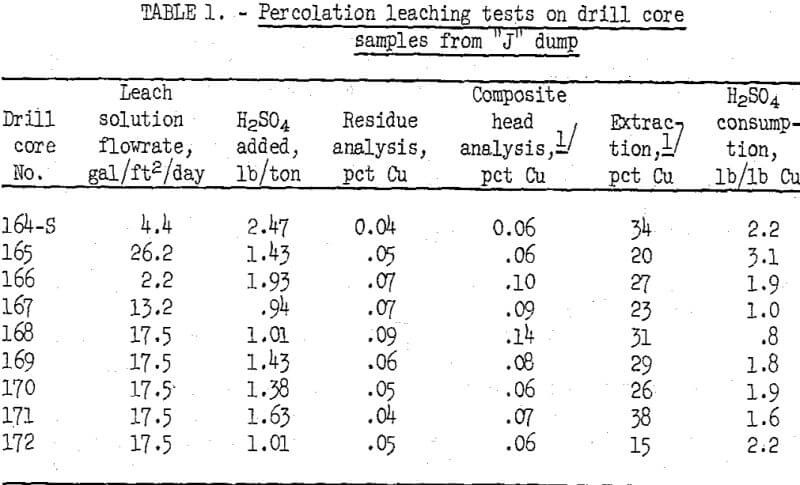
Laboratory-scale percolation leaching tests were made on the drill core samples in 4-inch-diameter by 36-inch-long glass tubes. Glass wool and small quartz pebbles were placed at the bottom of the ore column to prevent plugging of the drainage holes in the Buckner funnel which supported the loaded tube. Split samples of each 8-foot drill core interval from an individual drill hole were placed in a glass column in the same order in which the interval occurred in the dump. One liter of dilute sulfuric acid leach solution at pH 2 was fed to the top of each ore column. Leach solution flow rate was regulated to equal the rate of effluent discharge. A few inches of solution were maintained on the surface of the nine ore columns to simulate ponding on a dump. Copper-bearing liquor that drained from the columns was collected in flasks and recirculated continuously through the ore bed for 2 weeks. During this time, water was added as needed to make up for evaporation loss. Whenever the pH of the recirculating solutions exceeded 2.3 acid was added to lower solution pH to 2.0. At the conclusion of a 17-day leach cycle, each column was allowed to drain and dry for about 2 days to simulate the alternate wetting and drying procedure commonly employed in commercial practice. The pregnant copper solution was sampled for analysis, and the excess solution was discarded. The leaching and drying cycles were repeated three more times with fresh acid solution at pH 2 introduced at the beginning of each cycle. The tests were terminated at the end of four cycles (about 75 days). Summarized results of the acid leaching tests on the drill core samples from the “j” dump are given in table 1.
Addition of ferric sulfate to aid in oxidation and dissolution of the copper sulfide minerals was not necessary because the acid leaching solution dissolved some of the iron salts that had precipitated in the dump. The ferric iron content of the recycled leach liquors ranged from 0.2 to 2 grams per liter. Copper content of the leach liquors ranged from 0.1 to 2.7 grams per liter.
Analyses of the leach solution showed that 45 to 50 percent of the total copper extracted was dissolved in the first -cycle, 30 to 35 percent in the second cycle, 15 to 20 percent in the third cycle, and less than 5 percent in the fourth cycle. Leaching was discontinued after four cycles. Copper extractions ranging from 15 to 38 percent were obtained by percolation leaching of the core samples from the nine drill holes. The test results indicate that about 27 percent of the copper remaining in the “j” dump could be extracted under conditions in which compacted zones were broken up and made pervious to acid leaching solutions.
Leaching characteristics of the nine drill core samples were similar, except for the slower flowrate for samples 164-S and 166, which contained more fine material than the other samples. The slow flowrate did not adversely affect copper extraction.
A representative portion of the laboratory-leached residue from drill core 165 was examined to determine how the unleached copper occurred. A sample of the residue, which assayed 0.05 percent Cu, was ground to minus-65 mesh and fractioned with a heavy liquid of 2.9 specific gravity into sink and float products. A polished briquette of the sink product was prepared for metallographic examination. The residual copper occurred as chalcocite and covellite coatings on pyrite particles locked in the gangue minerals. Sporadic inclusions of chalcopyrite also were observed in the locked pyrite grains. Grinding to minus-65 mesh did not liberate the pyrite with which the copper minerals are associated.
Conclusions
Laboratory leach tests indicated that about 27 percent of the copper could be extracted from the “J” dump under ideal percolation leaching conditions using sulfuric acid solutions. Mineralogical examination of the residue from one of the laboratory-leached drill core samples showed that the residual copper occurred essentially as fine-grained chalcocite and covellite coating pyrite grains locked within gangue minerals. The residual copper in this mode of occurrence cannot be effectively reached by leach solution; hence, further leaching is unlikely to dissolve significant amounts of additional copper.
