Table of Contents
- Cement Copper Samples
- Processing Cement Copper to a Powder
- Flotation
- Variations in Operating Conditions
- Particle Size of the Feed
- Oxidation of the Feed Material
- Reagent Additions
- Conditioning Time
- Froth Depth
- Aeration
- Pulp Temperature
- Pulp pH
- Pulp Density
- Cleaner Flotation
- Flotation of Various Copper Samples
- Chemical Purification of Flotation Concentrate
- Digestion Studies
- Variations in Solution Composition
- Variations in Time and Temperature
- Copper Dissolution
- Purification of the Various Flotation Concentrates
- Reduction, Sintering, and Grinding
- Copper Powder Evaluation
- Process Variations
- Sintering Temperature
- Sintering Time
- Copper Powder Produced
- Cost Evaluation
Production of cement copper in the United States has been increasing during the past few years principally because more mine waste from open pit operations is being leached. During 1969 about 220,000 tons, or approximately 14 percent, of the Nation’s production of new copper was from leaching operations. The mine waste is leached with acid-ferric sulfate solution, and dissolved copper is recovered by cementation on iron. The cementation reaction is represented by the equation
Cu++ + Fe → Cu + Fe++………………………………………………………(1)
This reaction proceeds rapidly and efficiently to precipitate copper from the dilute leach liquors as cement copper.
In commercial practice, gravity launders, cone precipitators, or revolving drums are used for copper cementation. Shredded detinned cans are the chief form of iron used as the precipitant. The cement copper contains 60 to 90 percent Cu with oxygen the principal impurity, owing to partial oxidation during drying. Cement copper precipitates also are contaminated with minor amounts of silicate minerals, unreacted iron, stainless steel, and nonminerals such as rubber, fabric, paint, wood and paper associated with the shredded iron scrap. Standard practice is to smelt the impure cement copper in a reverberatory furnace along with copper sulfide flotation concentrates. The matte produced in the reverberatory furnace is converted to blister copper for electrolytic refining. Because of the increasing production of cement copper and the limited capacity at existing smelters, alternative processing routes should be considered.
As cement copper is composed of fine-sized particles and copper powder commands a premium price, the Bureau of Mines initiated research to develop a powder preparation process as an alternative route for utilizing cement copper. In prior investigations, copper powder was prepared from relatively pure cement copper that had been precipitated from leach solution with clean iron products. This report describes laboratory flotation, chemical treatment, and sintering techniques developed for the preparation of copper powder from industrially produced impure cement copper. Processing cost estimates also were made.
Cement Copper Samples
The cement copper samples used in this investigation were obtained from six western copper producers that leach low-grade mine dump material with acid-ferric sulfate solutions and recover copper by iron cementation methods. A detailed description of the leaching and cementation practices used is given in a recent Bureau of Mines Information Circular.
The cement copper samples were collected and shipped as wet pulps and, upon receipt, were screened on 10-mesh. The minus 10-mesh fraction was split into 400-gram samples with a rotary pulp splitter and stored in airtight plastic containers until needed. The plus 10-mesh material that contained less than 1 percent of the copper was not used for this investigation. This material could be treated by conventional smelting techniques.
Chemical Analyses
Chemical analyses of the minus 10-mesh fractions of the six cement copper samples are presented in table 1. Pretreatment of the assayed samples included filtering, washing, and oven drying at 100° C. During preparation, a small amount of copper and iron was oxidized. Three of the cement copper samples contained 91 to 95 percent Cu, whereas the other three contained 67 to 88 percent Cu. The three higher quality samples were carefully collected from the cementation launders. Care was taken to make sure that only freshly precipitated copper was obtained and that enough supernatant liquid was included to keep the copper from contacting the air. The history of the other three samples was not known, but subsequent examination indicated that the copper in samples Nos. 1 and 2 had been extensively oxidized to cuprous oxide. Apparently, these samples had been exposed to the air and partially dried during sampling. The impurity analyses shown in table 1 indicate the variability in composition of industrial cement copper. Iron is a major contaminant resulting from incomplete utilization of the scrap. The relatively high aluminum and insoluble content probably were caused by collodial material carried by the leach liquors into the precipitation launder.
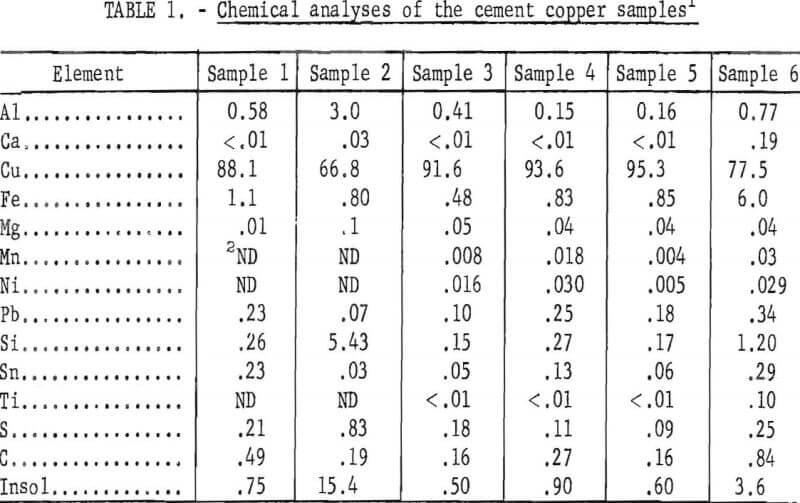
Sample Oxidation
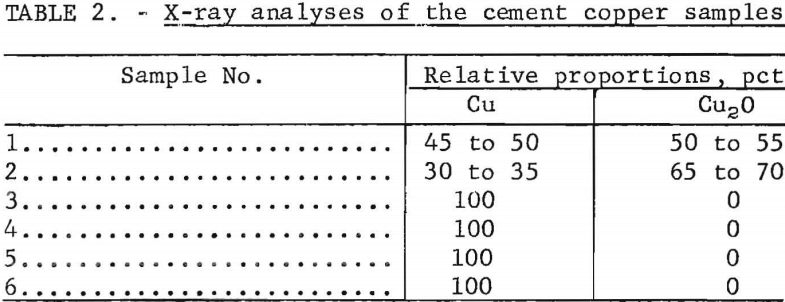
The degree of oxidation of the samples was determined by X-ray diffraction analysis. To prevent oxidation during drying, the samples were analyzed in a moist condition. Comparative analyses showed that the presence of moisture did not alter the X-ray results. The relative proportions of copper and cuprous oxide found in the various samples are shown in table 2. Cupric oxide, CuO, was not detected.
The X-ray analyses indicate that the copper in two of the samples was partly oxidized to cuprous oxide, and the remaining four contained only metallic copper.
Screen Analyses
The screen analyses of the six cement copper samples are presented in table 3.
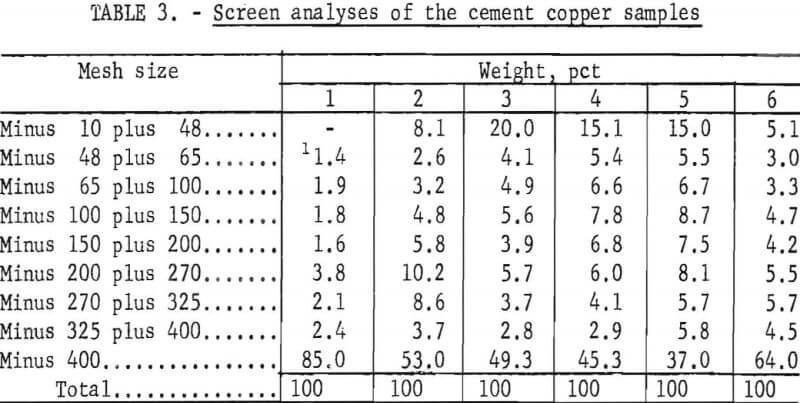
The screen analyses data shows that the particle size distributions varied among samples. Sample No. 1 contained the most fines, and 85 percent of the material was in the minus 400-mesh fraction; the coarsest sample, No. 5, contained only 37 percent minus 400-mesh fines.
Processing Cement Copper to a Powder
To obtain the desired purity and physical form, the cement copper was processed by (1) flotation to reject insoluble gangue material and unreacted iron, (2) chemical digestion in acidulated copper sulfate solution to lower the iron and aluminum content, and (3) hydrogen reduction at elevated temperature to remove oxygen and produce a sinter cake that, after grinding, resulted in a powder of suitable physical properties.
Flotation
Preliminary flotation tests indicated that upgrading of cement copper could be achieved with a dixanthogen reagent (Minerec A). This reagent acted as both a copper collector and a frother. The use of other reagents was unnecessary and usually detrimental to the upgrading process. The use of a frother decreased the selectivity of the flotation process, and the use of depressants for the gangue material also depressed some of the copper. Satisfactory flotation results were obtained by pulling four rougher concentrates and cleaning the combined rougher concentrates twice.
The total reagent consumption for this float was about 1.0 pound per ton. This appears to be high when compared with a common reagent consumption of less than 0.1 pound per ton for copper sulfide flotation. However, on the basis of the large proportion of copper floated, the consumption is about one-tenth that required for an equivalent amount of copper in sulfide flotation.
The flotation step was used primarily to separate the copper from the insoluble gangue material. With normal laboratory procedures, copper recoveries were in the range of 85 to 95 percent; the rejection of insoluble material ranged from 80 to 95 percent. Although these results were satisfactory from the standpoint of upgrading the cement copper, the results from replicate tests were not consistent enough to study the effects of variations in the operating conditions. Therefore, the flotation cell and the test procedure were modified to insure reproducible operating conditions. Figure 1 depicts the modified flotation cell, which includes a water jacket for temperature control, a mechanical froth scraper, and a flowrator for measurement of the air used in aeration.
Variations in Operating Conditions
The operating conditions studied were (1) particle size and degree of oxidation of the feed material,(2) rate and amount of reagent addition, (3) conditioning time, (4) aeration rate, and (5) the pulp level, temperature, pH, and density. Cement copper sample No. 6 was used throughout this phase of the investigation, and to insure consistent results, only samples from the same splitting operation were used for a particular series of tests. Unless otherwise stated, the minus 10-mesh sample was screened on 100-mesh, and the oversize was ground to minus 100-mesh in a ball mill and recombined with the undersized, and the combined products were split into flotation-size samples.
In addition to a minus-100-mesh flotation feed, the selected conditions from which variations were made were as follows; (1) Four stages of rougher flotation using 0.252, 0.144, 0.144, and 0.144 pound per ton of dixanthogen collector, respectively; (2) conditioning time of 2 minutes and flotation time of 3 minutes for each rougher; (3) pulp level adjusted to give a froth column of 4.5 centimeters; (4) aeration rate of 4.7 liters of air per minute per

liter of pulp; (5) pulp temperature 24.0±0.5° C (6) natural pulp pH (5.8 to 5.0); and (7) an initial pulp density of 22 percent solids. Results from a single test using these conditions are given in table 4.
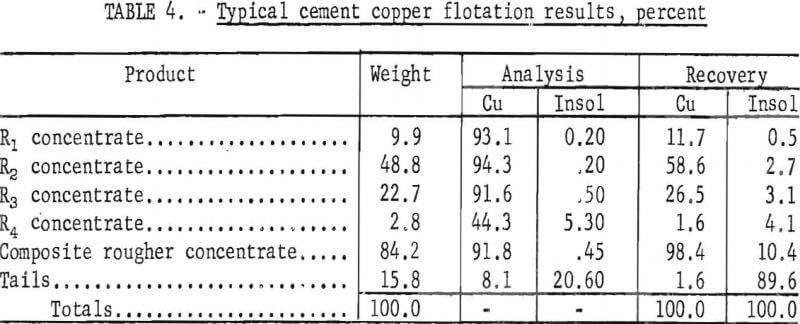
The initial analysis of the flotation feed, in percent, was 77.5 Cu and 3.6 insoluble material. The cement copper was upgraded to a product analyzing 91.8 percent Cu and 0.45 percent insoluble material with a copper recovery of 98.4 percent. By omitting the small amount of low-grade fourth rougher concentrate, the composite of the first three concentrates contained, in percent, 93.4 Cu and 0.28 insoluble material, and copper recovery was 96.8 percent. The disproportionate amount of insoluble material that reported with the fourth rougher concentrate was characteristic of cement copper flotation. However, in most of the subsequent tests, four rougher concentrates were pulled to determine the influence of the variables on copper recovery and gangue rejection.
Particle Size of the Feed
The size of the cement copper feed ranged from minus 10-mesh to minus 100-mesh. Both single and multiple stage grinding was used. The results for all the tests were essentially the same and did not vary with the particle size of the feed material. Copper recoveries were 97 to 98 percent and insoluble rejections were about 93 percent. These results were obtained by batch flotation tests, and the physical problems associated with large dense copper particles in a continuous flotation circuit were not determined.
Oxidation of the Feed Material
Because cement copper readily oxidizes and two of the six cement copper samples obtained for this investigation were already partly oxidized, it was deemed advisable to determine the effect of oxidation on the flotation process. An oxidized cement copper pulp was prepared by air drying unoxidized sample No. 6 at room temperature. X-ray analysis showed that the sample had been altered from 100 percent elemental copper to 70 percent elemental copper and 30 percent cuprous oxide. In comparative flotation tests, a more voluminous froth was produced with the oxidized sample than with the unoxidized sample. The overall copper recovery and the amount of insoluble material floated was unchanged by the oxidation of the sample. However, the bulk of the copper was floated in the first and second rougher stages with the voluminous froth, whereas without prior oxidation, most of the copper floated during the second and third rougher stages (table 4).
Reagent Additions
The established schedule for adding the dixanthogen reagent to the flotation cell was 0.252 pound per ton in the first rougher and 0.144 pound per ton in each of the ensuing three roughers. The larger amount was needed in the first rougher to provide sufficient froth for flotation, but even with 0.252 pound per ton, only a small amount of copper floated. A series of parallel tests was conducted to determine if reagent economy could be affected. The amount of reagent used for the first rougher was maintained constant at 0.252 pound per ton, and 0.114, 0.108,and 0,072 pound per ton were alternatively used for the remaining three rougher stages of different tests. The total amounts of reagents used were 0.684, 0.576, and 0.468 pound per ton, and the respective copper recoveries were 98.4, 94.0, and 66.0. Therefore, to maintain high copper recovery, the amount of reagent used could not be reduced.
Conditioning Time
The flotation feed was conditioned in the flotation cell with dixanthogen reagent for periods of 1, 2, 4, and 6 minutes for each rougher concentrate. The tests indicated that this factor was not critical to cement copper flotation. However, with periods of less than 1 minute, froths did not appear immediately upon aeration. During the flotation period, the froths gradually improved to give a satisfactory float
Froth Depth
Control of the froth depth was accomplished by adding water to a measured pulp level before each stage of rougher flotation and by not adding water during the stage. The initial froth depths and results of the flotation tests are tabulated in table 5.
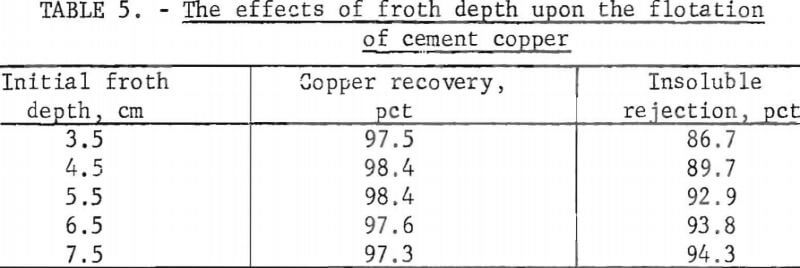
The data in table 5 show that the copper recovery did not vary significantly with varying froth depths, but the insoluble rejection increased with increased froth depth. Apparently, with increased froth depth, more time was allowed for differential draining in the froth and concentrate cleaning was improved. Thus, proper selection and control of froth depth will materially aid the upgrading of cement copper by flotation.
Aeration
For variations in the rate of aeration, compressed air was connected through a pressure regulator and a flowmeter to the aeration mechanism of the flotation cell. The airflow was adjusted to a predetermined reading on the meter and maintained constant during each flotation test. The aeration rates and flotation results are given in table 6.
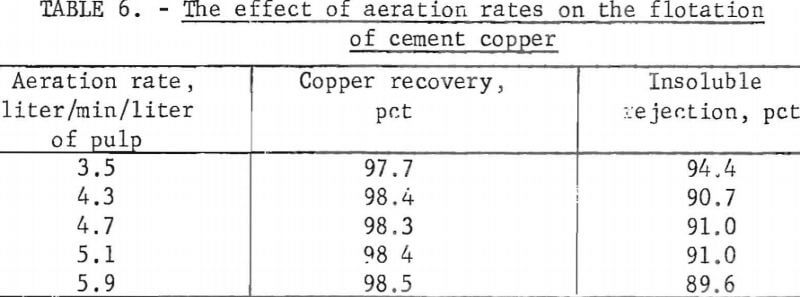
The test results showed that copper recoveries for the various aeration rates were essentially the same, but the insoluble rejection decreased with increased rates of aeration. The grade of the first three rougher concentrates for the various tests were all about the same, but with increased aeration the insoluble content of the fourth rougher materially increased, and this decreased the overall insoluble rejections.
Pulp Temperature
Constant-temperature water was circulated through a water jacket fitted around the flotation cell to regulate and control the pulp temperature during flotation. Without temperature control, the pulp temperature increased as much as 5° C during four stages of rougher flotation. With temperature control, variations were within ½° C. Copper recoveries and insoluble rejections obtained at different pulp temperatures are presented in table 7.
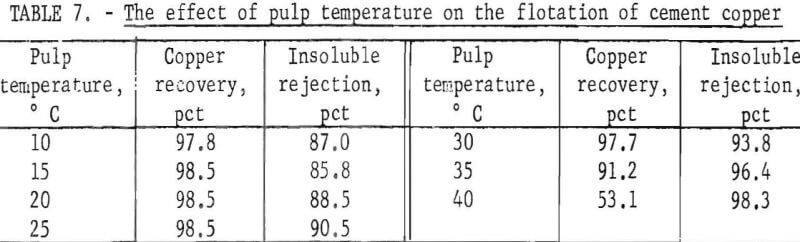
Cleaning takes place within a froth by differential draining of pulp from the bubbles. In addition, when the top layer of bubbles breaks, their load is transferred to the bubbles below and a crowding effect is produced. The crowded particles compete for places on the bubble surface, and the result is a heavy, mineralized, clean froth. It was noted during this series of tests that as the temperature increased the surface bubbles were less enduring. The gradual increase in insoluble rejection as the temperature increased indicated that the crowding effect was increased and cleaner froths resulted. However, at a pulp temperature of 40° C, the froth was not persistent enough to achieve an acceptable copper recovery.
Pulp pH
The natural pulp pH for the cement copper sample used in this series of flotation tests was 5.8. Lower pH’s were obtained by adding H2SO4 to the pulp; CaO was added to attain higher pH’s. The variations in pulp pH and the results on copper recovery and insoluble gangue rejection are shown in table 8.
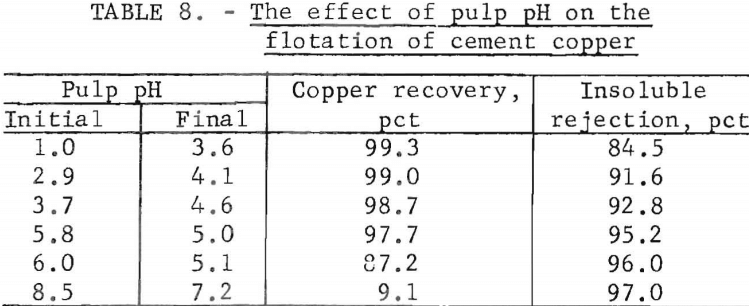
The acid requirements to obtain the initial pH values of 1.0, 2.9, and 3.7 were 230, 115, and 23 pounds, respectively, of H2SO4 per ton of cement copper feed. The respective CaO additions needed for pulp pH values of 6.0 and 8.5 were 2.5 and 10.0 pounds per ton. The conditions prevailing during flotation vitiated attempts to maintain a constant pH. The pulp pH decreased from high initial values and increased from low initial values. This trend indicated that the aerated pulp was buffered at a pH of about 5.0. However, unlike solutions that are buffered instantaneously, the pulp required time to reach equilibrium.
The frothing characteristics of the pulp varied with the pulp pH. At a low pH the froth was voluminous and copper and some gangue were readily floated. The converse was true at a high pH. At the natural pH of 5.8 declining to 5.0, the froth was sufficient to achieve a copper recovery of 97.7 percent and not so abundant as to interfere with the rejection of the gangue material.
Pulp Density
Upgrading cement copper by flotation involved floating the major constituent in a concentrate while leaving the unwanted, minor constituent behind. About 80 to 85 percent (by weight) of the cement copper reported in the concentrate and wide variations between initial and final pulp density were encountered during a single test. The results of cement copper flotation from pulps of various densities are shown in table 9.
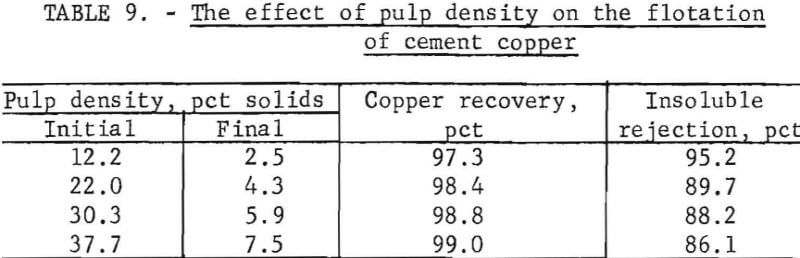
The tabulated copper recoveries show that for variations of initial pulp density from 12.2 to 37.7 percent solids, the copper recovery increased by only 1.7 percent. Of more significance was the variations in insoluble rejection with increased pulp density. An increase in the initial pulp density from 12.2 to 22.0 resulted in a relatively large decrease in insoluble rejection, and further increases in pulp density resulted in nominal decreases in insoluble rejection. Higher grade concentrates were attained with the more dilute pulps.
Cleaner Flotation
The upgrading process was further improved by cleaning the rougher concentrate. The cleaning procedure consisted of combining the four rougher concentrates and refloating them in two cleaner stages. An additional 0.144 pound per ton of reagent was used in each cleaner stage and the other flotation conditions were the same as used for the rougher flotation Results of the cleaner flotation tests showed that the final cleaner concentrate contained 97.3 percent of the copper and that 96.9 percent of the gangue was rejected. The cleaner concentrate analyzed 93,3 percent copper and 0.10 percent insoluble material. Cleaner flotation was necessary to reduce the insoluble content to a level that, would be acceptable for a copper powder.
Flotation of Various Copper Samples
The general flotation procedure previously established was applied to all six of the industrial cement copper samples with minor variations in reagent amounts and addition schedules to achieve optimum results. Variations, which were established independently for each sample, were based on operator judgments and test results. High copper recovery and high insolubles rejection were the main criterion used. Rejection of the other impurities was an additional benefit Results of the flotation tests are summarized in table 10.
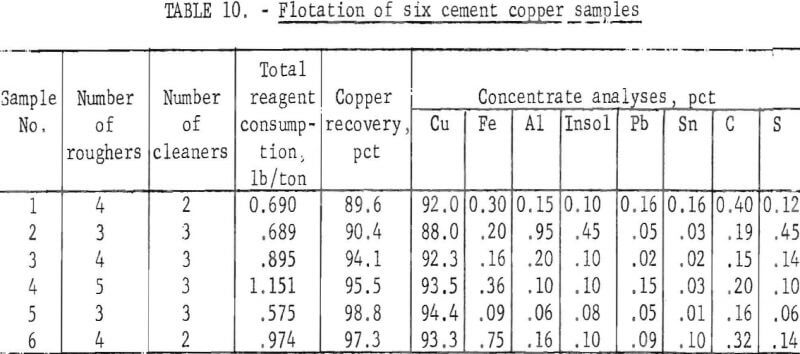
Examination of the data in table 10 shows that good copper recoveries were attained with each copper sample. The grades of the cleaner concentrates were between 88.0 and 94.4 percent copper, and five of the six concentrates had insoluble contents of 0.1 percent or less. Microscopic inspection of the various concentrates showed essentially complete rejection of the discrete gangue minerals; the remaining insoluble material present was collodial silica. The abnormally high insoluble content of 0.45 in concentrate No. 2 was attributed to the excessive amount of insoluble gangue (15.4 percent) in the cement copper feed, Likewise, the high iron content of concentrate No. 6 was attributed to the high iron in the initial sample.
Comparison of the analyses of the cement copper samples (table 1) with the concentrate analyses shows that all of the listed impurities were decreased by the flotation process. The iron, aluminum, insoluble material, lead, and tin were materially decreased, but the sulfur and carbon contents were only moderately decreased.
Chemical Purification of Flotation Concentrate
The cement copper flotation concentrates were further purified by selectively dissolving some of the impurities in acid solution. The major impurities universally present were iron, aluminum, carbon, and insolubles; in some instances lead, tin, and sulfur were present in amounts greater than 0.10 percent. Only the iron and aluminum responded readily to the chemical purification. Because of impurity variations in flotation concentrates from the six cement copper samples, the general effects of operating conditions upon purification were investigated rather than optimizing the conditions for a single concentrate.
Digestion Studies
A series of tests was made to determine the effect of digestion solution composition, solution pH, time, and temperature upon purification and dissolution of the upgraded cement copper. The concentrate used was derived from cement copper sample No. 1 and contained, in percent, 0.45 Fe and 0.26 Al. This concentrate was produced with less than optimum flotation conditions and contained more iron and aluminum than previously reported in table 10. However, the differences were small and did not alter the outcome of the investigation.
Variations in Solution Composition
Cement copper flotation concentrates were digested in acidulated copper sulfate or sulfuric acid solution. The invariant conditions were digestion for 60 minutes at 50° C in a slurry of 10 percent solids without agitation. After digestion, the pulp was filtered and the residue washed and dried at 100° C. Some oxidation occurred during drying but most of the copper analyses ranged from 90 to 95 percent. The composition of the digestion solutions with the test results are listed in table 11.
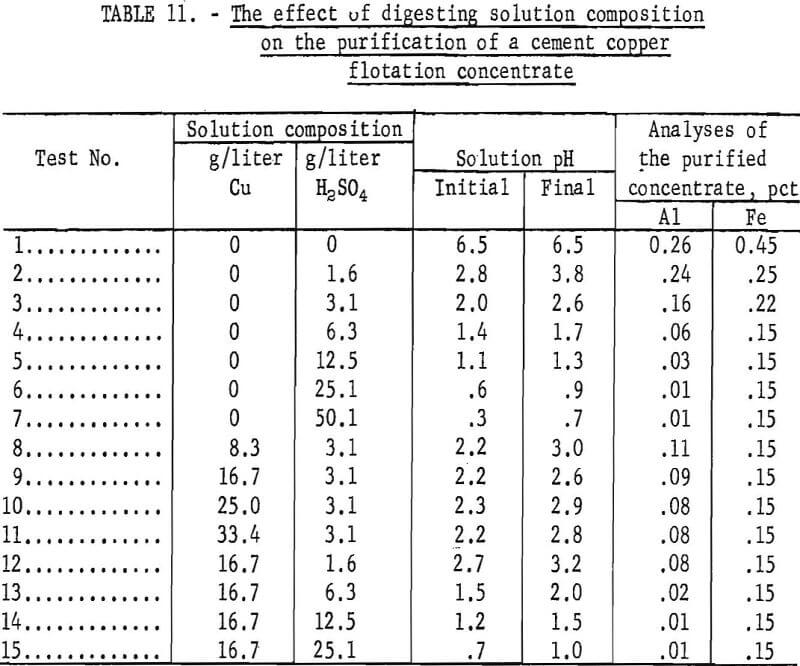
The aluminum and iron analyses of the undigested concentrate and the natural pH of the concentrate slurry are listed as test 1 of table 11. Tests 2-7, inclusive, comprise a series of digestions with increasing acid concentrations. The data indicate that a pH of 0.6 to 0.9 (25.1 grams H2SO4 per liter) was required to minimize the aluminum content, whereas at pH 1.4 to 1.7 (6.3 grams H2SO4 per liter) the iron content was minimum. A more acidic medium was needed to dissolve the aluminum compounds than the iron compounds.
Tests 8-11 were a series of digestions with increasing Cu++ concentrations at pH 2.2 to 3.0. Comparison of tests 3 and 8 shows that when digesting at pH 2.0 to 3.0, the use of 8.3 grams Cu per liter in digesting solution will improve the dissolution of both the aluminum and iron. Without copper in solution the respective aluminum and iron analyses were 0.16 and 0.22, and with copper in solution, the aluminum and iron contents were reduced to 0.11 and 0.15.
Tests 12-15, inclusive, comprise a series in which the copper content of the solution was maintained at 16.7 grams per liter, and the acid varied from 1.6 to 25.1 grams per liter. The data showed that a solution containing 16.7 grams Cu per liter and an initial pH of 1.2 to 1,5 was required to achieve a minimum (0.01 percent) aluminum content. With acid alone (test 6), a pH of 0.6 to 0.9 was required to attain the same low aluminum content.
Variations in Time and Temperature
The effect of time and temperature on purification of the cement copper flotation concentrate was investigated. A digestion solution that contained 16.7 grams Cu per liter and 12.5 grams H2SO4 per liter was used. Tests were made at 30° C and the time ranged from 60 minutes to 5 hours; at a pulp temperature of 50° C, the time ranged from 30 minutes to 2 hours. The tests showed that only 30 minutes contact time was necessary to achieve adequate purification at 50° C,whereas 180 minutes was required when digesting at 30° C. At either condition, the aluminum content of the cement copper float concentrate was reduced from 0.26 to 0.01 percent, and the iron was reduced from 0.45 to 0.15 percent.
Copper Dissolution
Metallic copper, or cuprous oxide, should not dissolve appreciably in the acidic solutions used for the digestion study. However, cement copper, did dissolve during digestion, and the extent of dissolution increased with increased time and temperature, and decreased with pH. At a nominal temperature and time of 50° C and 60 minutes, the amount of copper that dissolved in various sulfuric acid digestion solutions is shown in table 12.
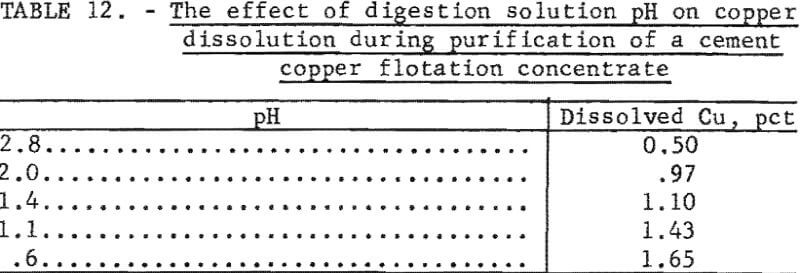
Apparently, the cement copper was partially oxidized during processing; this oxide dissolved during digestion. Therefore, to minimize copper dissolution, the pH of the digesting solution should be as high as possible that is compatible with satisfactory purification.
Heretofore, the cement copper concentrates were digested without the aid of stirring. Additional tests with stirred pulps showed that the rate of purification was increased, but the amount of copper dissolved also increased. With a solution pH of 0.6, about 4 percent of the copper dissolved with stirring as compared with 1.65 percent without stirring. Therefore, in subsequent work the cement copper concentrates were digested without stirring at a pH of 1.5 to 2.0 to prevent excessive, dissolution of the copper.
Purification of the Various Flotation Concentrates
Each of the six cement copper samples was floated to obtain a cleaner concentrate and the concentrate digested without stirring to obtain a purified product. The digestion solution contained 16.7 grams Cu per liter, 6.3 grams H2SO4 per liter (pH 1.5 to 2.0), the digestion time was 60 minutes, the temperature was 50° C, and the pulp density was 10 percent solids. The analyses of the resulting purified flotation concentrates are shown in table 13.
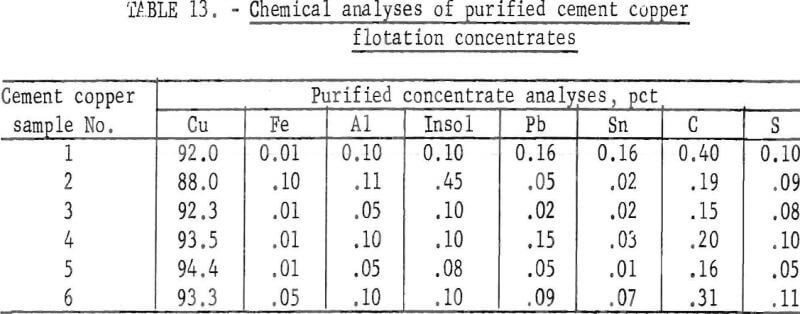
A comparison of the chemical analyses of the various flotation concentrates (table 10) with the analyses presented in table 13 indicates that aluminum and iron were substantially decreased by the digestion procedure and that the remaining impurities were essentially unchanged. However, cement copper sample No. 2 represents an exception because the flotation concentrate contained 0.45 percent S, and this was reduced to 0.09 percent by the digestion procedure. Apparently, this concentrate contained sulfate salts that were dissolved by the acid treatment. The relatively high insoluble gangue (0.45 percent) in sample No 2 is indicative of the large amount of this component in the initial cement copper sample (15.4 percent). The principal impurity remaining in the concentrates was carbon, which was derived from the wood and other carbonaceous trash associated with, the iron scrap used for cementation of the copper.
Reduction, Sintering, and Grinding
The dried cement copper concentrate from chemical purification was not of satisfactory quality for use as a copper powder. The concentrate was partially oxidized, which would interfere with cohesion of the particles during a pressing and compacting operation. The purified copper also contained a large proportion of fine-sized particles when compared with commercial copper powder. To overcome these deficiencies, the concentrates were reduced and sintered in a hydrogen atmosphere and the sinter cake ground to a powder. Reduction of the copper oxides was essentially complete when the conditions were met for adequate sintering. Therefore, during this phase of the investigation, the effect of sintering conditions on the physical properties of the powder was emphasized. The grinding procedure was purposely held invariant so that powder properties would be comparable.
Copper Powder Evaluation
The physical properties used to define a copper powder are apparent density, flow characteristics, particle size, and particle size distribution. These properties were measured using as a guide methods established by the Metal Powder Association. The particle size and particle size distribution of the various powders were determined by using Tyler standard screens and a mechanical shaking device. The flow characteristics of the ground powder were determined with a Standard Hall Flowmeter and evaluated by the number of seconds required for 50 grams of sample to pass through a calibrated orifice. The flowmeter was equipped with a calibrated 25-cubic-centimeter receiving cup that was used for determining apparent densities.
Definite specifications for the many different uses of copper powders are nonexistent. In general, the evaluation of a copper powder requires processing a test lot of the powder to the desired end product. If satisfactory, the chemical and physical properties of the test lot powder are determined and these become the specifications.
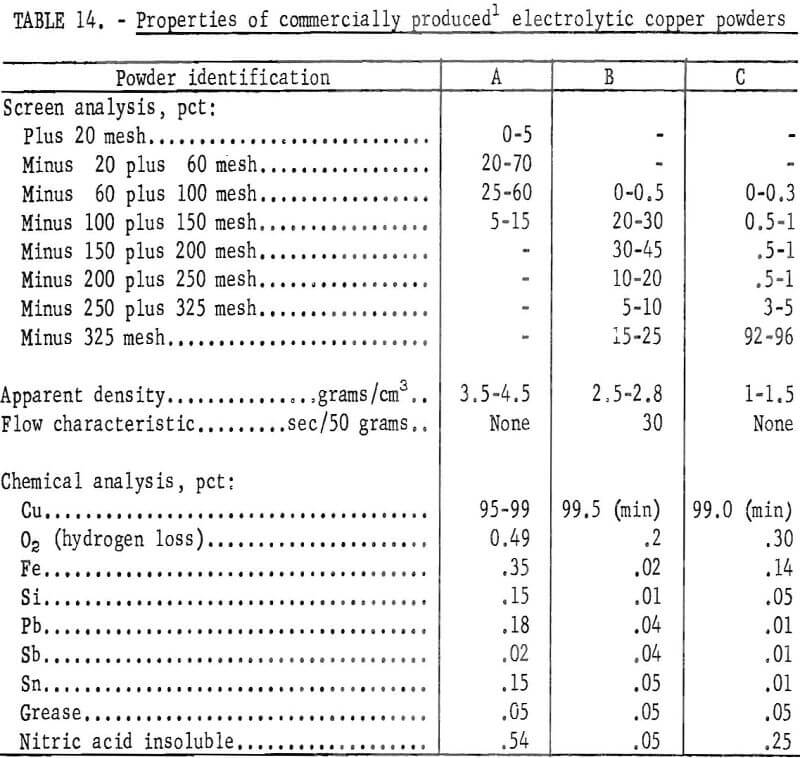
Making a complete evaluation of copper powders by end use was not feasible within the scope of the investigation. Instead, the chemical and physical properties of the copper powders prepared from cement copper were compared with the similar properties of commercial powders. The properties of three different types of commercial copper powders are given in table 14.
Process Variations
The sintering procedure consisted of placing the copper sample in a pre-heated furnace tube, purging the system with nitrogen, and maintaining a hydrogen flow over the charge during the reduction and sintering period. At the completion of the test, nitrogen was again introduced and the tube removed from the furnace and cooled with a water spray. The sinter cake was ground to a powder in a swing hammer laboratory impact mill. The resulting powder was screened at 60 mesh and the oversize reground.
Sintering Temperature
Purified cement copper flotation concentrates were reduced and sintered at various temperatures and the physical properties of the resulting copper powders determined. Ninety minutes sintering time was used for each temperature. The sintering temperatures and test results are listed in table 15.
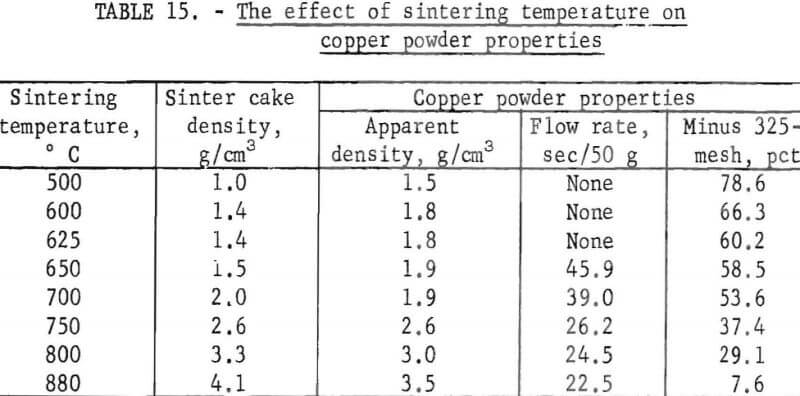
The data show that as the sintering temperature as increased from 500° to 880° C, the density of the sinter cake increased from 1.0 to 4.1 grams per cubic centimeter. As the density of the sinter cake increased, the apparent density, the flow rate, and particle size of the resulting powders increased. Thus, control of the sintering temperature offers a means of controlling the physical properties of the copper powder.
A comparison of the physical properties of the copper powder obtained by sintering at 700° and 750° C with the properties of commercial copper powders listed in table 14 showed similar characteristics. Copper powders produced from cement copper should be acceptable for powder metallurgical applications. However, for final evaluation the deportment under actual pressing and sintering conditions must be considered.
Sintering Time
Sintering tests were made using various sintering times at a temperature of 750° C. The times used and powder properties are reported in table 16.
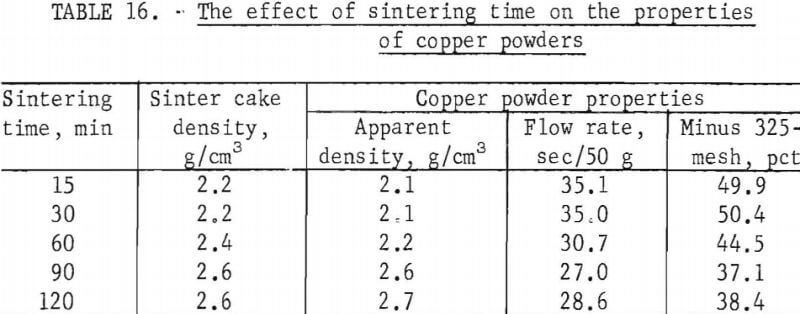
Inspection of the test data indicates that powder properties vary with increased sintering time similar to the trend noted for increased temperature. However, with time, the variations in powder properties were not extensive. Control of sintering time will be necessary to produce characteristically identical powders and offers an alternate method for controlling minor variations in powder properties.
Copper Powder Produced
Copper powder was prepared from five of the previously described cement copper samples. Sample No. 2 was completely consumed during the flotation and chemical digestion test work. Another sample from the same source differed extensively in chemical analyses and, therefore, was not used. The essentials of the process used to prepare copper powder from the five remaining samples were (1) rougher and cleaner flotation with Minerec A, (2) digestion in acidulated CuSO4 solution for 1 hour at 50° C, (3) filtering and washing, (4) drying at 100° C, (5) reduction and sintering for 1 hour at 700° C, and (6) grinding to minus 60 mesh. The chemical and physical properties of the prepared copper powders are given in table 17.
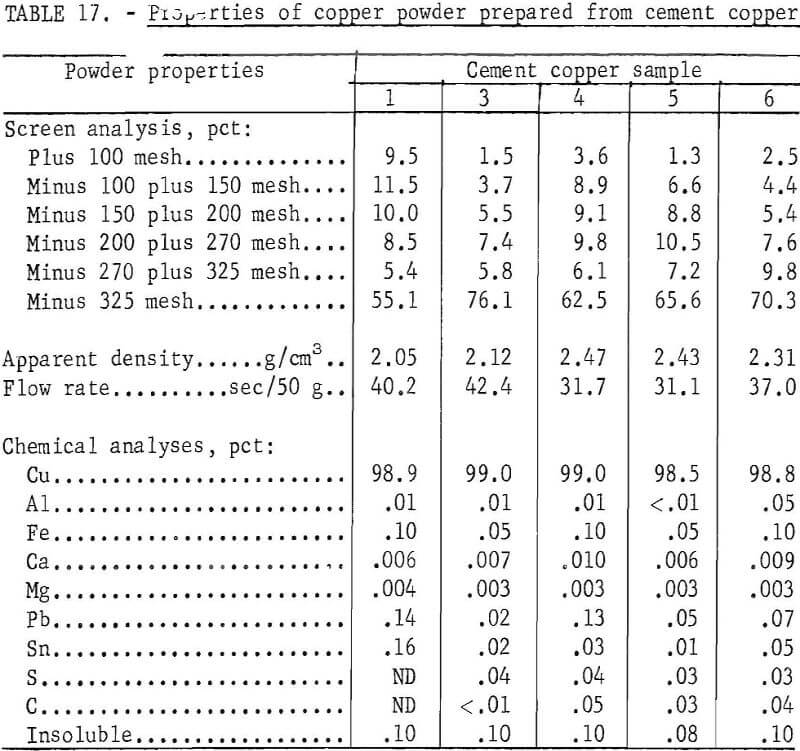
The copper powder prepared from each of the cement copper samples had physical and chemical characteristics similar to those of commercially available powders shown in table 14 and, therefore, should be suitable for powder metallurgical applications. However, characteristically identical powders were not produced from the different cement copper samples. A commercial application of this method of manufacturing powders will need to include sampling and testing along with mixing and blending to compensate for variations in cement copper feed material and to insure a uniform powder product.
A comparison of the powders produced (table 17) with chemically purified flotation concentrates (table 13) shows that in each case, the copper analysis was materially increased and that both the carbon and sulfur analyses were decreased. Apparently, hydrogen reduction of the copper oxides was complete, the carbonaceous material reacted with copper oxide and was evolved as carbon monoxide or carbon dioxide. Most of the small amount of remaining sulfur reacted with the hydrogen and was removed as hydrogen sulfide.
Cost Evaluation
An economic evaluation of the process was made. Laboratory data was used to estimate equipment size and costs for plants that produce 10 and 25 tons per day of copper powder. Cement copper screening and sinter cake grinding operations were designed to operate 1 shift per day and the remainder of the plant to operate 3 shifts per day for a 7-day week.
Construction costs and the total fixed capital costs for each unit operation were estimated by means of standard factors for similar unit operations. Fixed capital costs for facilities and utilities were estimated to be 10 and 12 percent, respectively, of the total fixed capital costs for the unit operation.
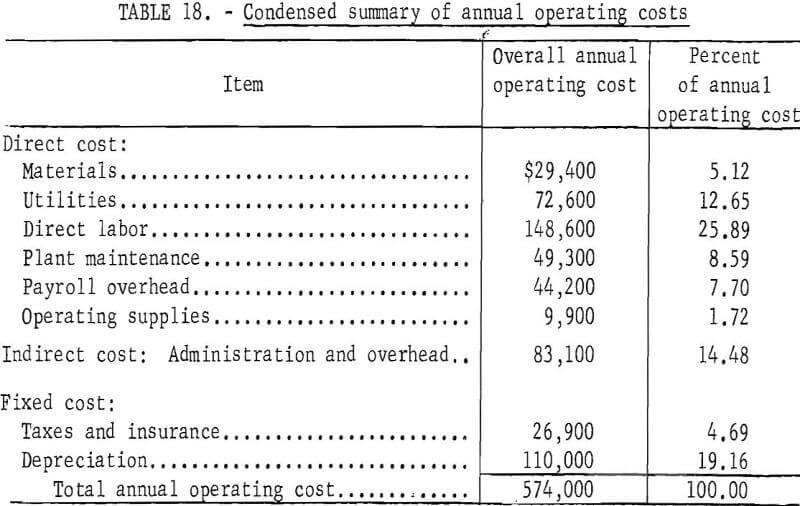
Annual operating costs were estimated for each unit operation and for the overall cement copper plant. No cost was included for the cement copper feed to the plant. Utilities were charged to the unit operations using them at a rate that includes the base rate to the plant plus a charge for distribution within the plant. A total of 18 men were assumed necessary to operate the plant. Maintenance costs were estimated by means of standard factors for each unit operation. Additional standard factors were used for other direct and indirect costs. Administration and overhead charges to the unit operations were adjusted to include a proportionate share of the operating costs of the facilities. Taxes and insurance were estimated to be 2 percent of the fixed capital costs without interest. The plant was depreciated over 12.5 years.
A plant to produce 10 tons of copper powder per day required $795,000 fixed capital, and $100,000 working capital, and entailed an annual operating cost of $417,000. The operating cost is equivalent to 6 cents per pound of copper powder.
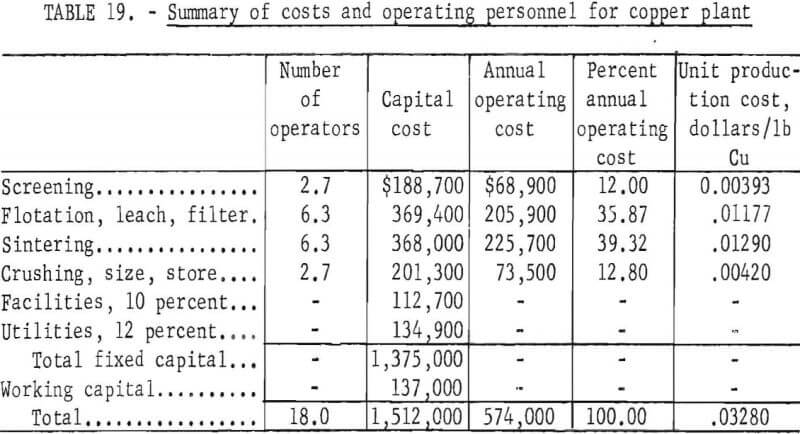
An increase in production to 25 tons of copper powder per day required $1,375,000 fixed capital, and $137,000 working capital, and entailed an annual operating cost of $574,000. The operating cost in this case is equivalent to 3.3 cents per pound of copper powder. An increase in production capacity generally results in some decrease in unit production cost. With small plants such as these, an increase in capacity results in much more efficient use of manpower and, therefore, shows a marked decrease in unit production cost. Summaries of costs and operating personnel are given in tables 18 and 19 for the 25-ton-per-day plant.
Conclusions
The laboratory investigation demonstrated that industrial-grade cement copper can be processed to copper powder. The processing scheme included (1) flotation to reject a major proportion of the gangue, (2) chemical digestion to decrease the aluminum and iron content, (3) gaseous reduction to reduce the oxides, (4) sintering to agglomerate the fine sized particles, and (5) grinding the sinter cake to a powder. Copper powders prepared from various source materials were comparable in quality and physical properties to commercially available copper powders.
In addition, the study indicated that the chemical purity and physical properties of the powder can be controlled by variations in the processing conditions. Variations in the flotation procedure and chemical digestion procedure influence the chemical purity; variations in sintering time and temperature markedly affect the physical properties.
Based on the results of the small-scale tests, an economic evaluation of the process was made. A small plant that produced 10 tons of copper powder per day required $795,000 fixed capital, $100,000 working capital, and an annual operating cost of $417,000. The operating cost was equivalent to 6 cents per pound of copper powder. A larger plant that produced 25 tons per day required $1,375,000 fixed capital, $137,000 working capital, and the annual operating cost was $574,000. This operating cost per pound of copper powder was 3.3 cents. These costs appeared modest when considering that copper powder is a premium product and commands a price normally about 17.5 cents per pound over that of electrolytic cathode copper.
