Table of Contents
Silver was one of the first metals known to man, and metallurgical processes for the recovery of silver from ores date back into antiquity. Mercury amalgamation and retorting to recover metallic silver from slags, mine tailings, and scrap were in common use by the end of the 15th century. In 1557, the Patio process was used in Mexico. This process involves the amalgamation of silver formed by the reaction of silver minerals with sodium chloride and copper sulfate. The Washoe pan amalgamation process, a modification of the Patio process, was used in 1861 for the Comstock Lode ores. The process consisted of treating finely ground ore with steam and adding mercury, sodium chloride, copper sulfate, and sulfuric acid to the pulp. The silver was recovered as an amalgam. For more complex ores, a salt roast was employed prior to using the Washoe process. This salt roast treatment, known as the Reese River process, was used at Austin, Nev., in the late 19th century. By the start of the 20th century, cyanidation had replaced all amalgamation-type processes, except for use with ores containing coarse metallic silver. The cyanide technique was used to rework many of the tailings; however, silver recovery from some of the more refractory tailings was frequently less than 50 percent.
Mechanical losses of mercury during processing by amalgamation techniques can average 2 pounds per ton of ore, and frequently the tailings contain both silver and mercury. A portion of the mercury in the tailings exists as silver amalgam. The average amount of silver in these tailings is in the 3- to 5-ounce-per-ton range. Corresponding mercury content varies between a few tenths of a pound and 5 pounds per ton. The use of techniques, such as cyanidation, for removing silver from these tailings typically results in 50 to 80 percent silver extraction. Mercury extraction is very low because metallic mercury is only slightly soluble in cyanide. In aggregate, these tailings represent a source of several million ounces of silver; however, a new metallurgical technique is needed to obtain reasonable silver recovery from these refractory materials.
An electrooxidation process originally developed by the Bureau of Mines for pretreatment of carbonaceous gold ores was effective for dissolving sulfide mercury ores. Subsequent investigation indicated that the process could be successfully applied to a variety of other sulfide and refractory minerals to dissolve the metal values principally as chloro complexes.
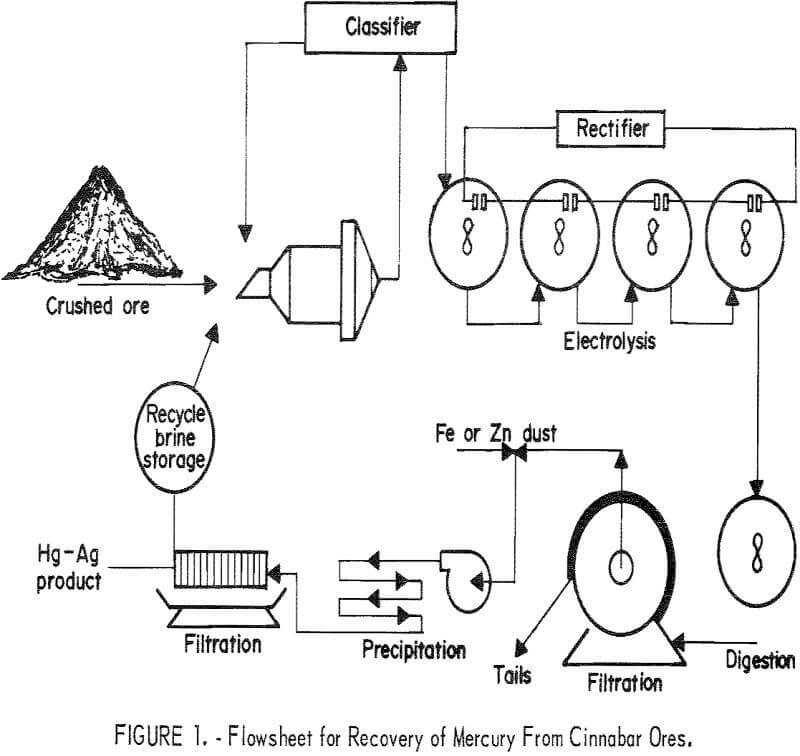
A conceptual flow sequence for electrooxidation as it was applied to extraction and recovery of mercury from cinnabar ores is presented in figure 1. The procedure consists of crushing and grinding the ore, forming a pulp with brine solution, and electrolyzing the pulp to oxidize the minerals to form brine-soluble compounds. The overall reaction for dissolution of cinnabar is shown in the following equation:
HgS + 4OCl- → HgCl4²- + S04²-……………………………………………….(1)
Conventional liquid-solid separation techniques are employed to obtain a relatively clear pregnant solution. The tails are pumped to a tailings pond, and the brine solution is recycled to the plant from the pond. The only consumption of salt is that which is entrained with the compacted tailing in the tailings pond. This salt loss ranges from 20 to 80 pounds per ton depending on the settling character of the ore. Because of possible pollution to the environment from the soluble mercury and sodium chloride, the tailings pond should be sealed to prevent leakage. Precipitating the mercury as an amalgam by an active metal such as zinc or iron produces barrens containing less than 0.1 parts per million mercury.
This Bureau of Mines report describes the investigation of the electrooxidation process as applied to the treatment of tailings for recovery of the
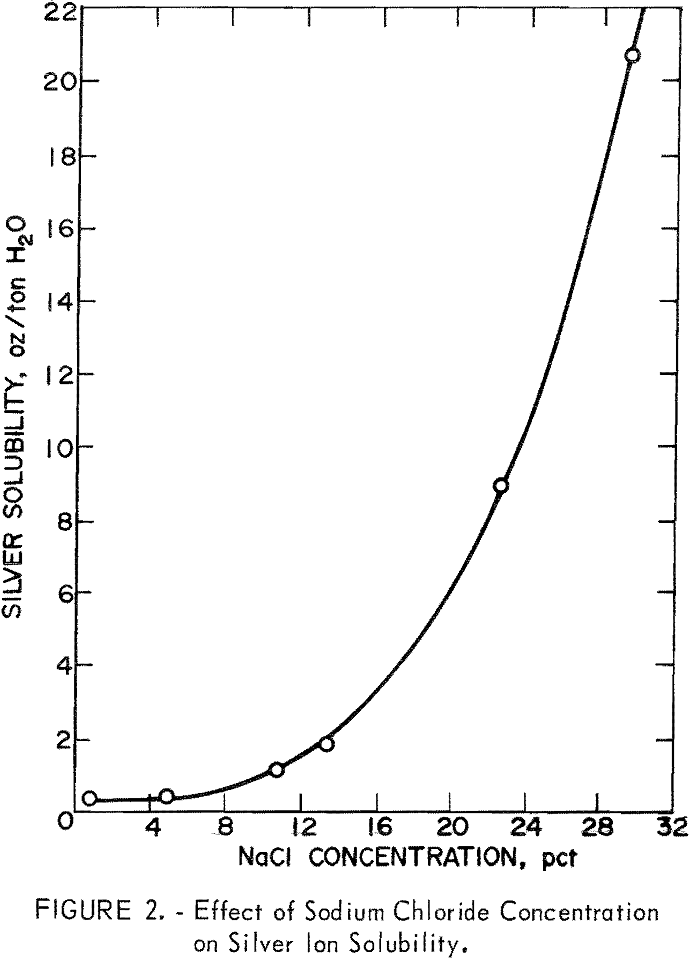
silver and mercury values. The oxidation in the presence of chloride ion would convert the silver mineral to silver chloride, which is soluble in brine solution as the silver tetrachloride complex (AgCl4³-). As shown in figure 2, the solubility of silver chloride as the chloro complex is highly dependent on chloride ion concentration. A concentration of at least 15 percent sodium chloride is required to dissolve 2.5 ounces of silver per ton of water, and a 29-percent solution of sodium chloride is required to dissolve 20.6 ounces of silver per ton of water. The data indicate that a salt solution in the 20-percent range would be necessary to obtain favorable solubility of silver in solution.
The silver minerals in tailings that have undergone a thorough salt roast would not be expected to be oxidized further by the electrooxidation technique. However, an electrooxidation treatment could complete oxidation started by either nature or the salt roast. Mercury in the amalgamation tails occurs as amalgam and synthesized sulfides and would be solubilized as the tetrachloro complex of mercury.
Materials and Procedures
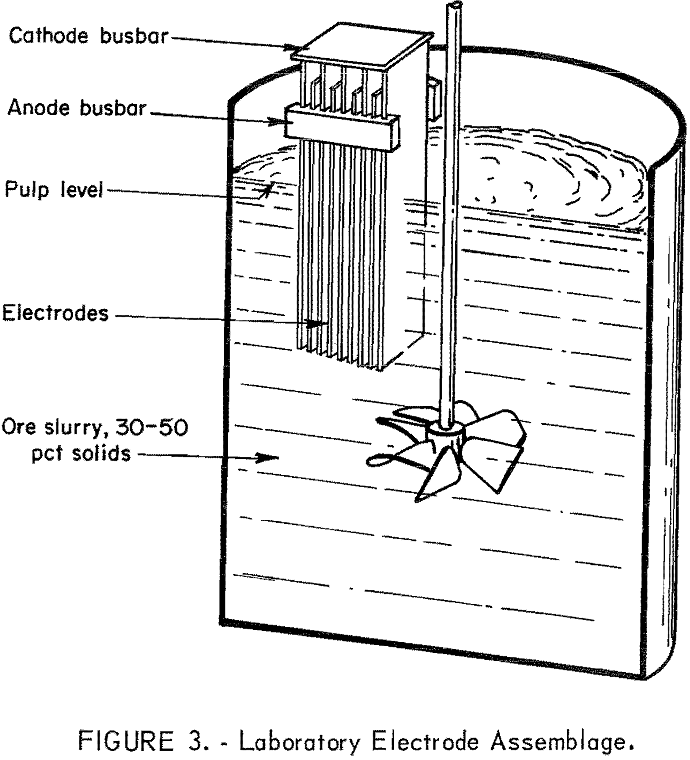
Tailings used in these experiments were from Unionville, Nev, Austin, Nev, and Zacatecas, Mexico (table 1). Experimental procedures consisted of (1) pulping 4 pounds of ground tailings and 540 grams of sodium chloride with 2.7 liters of water in a 4-liter beaker equipped with a stirrer; (2) placing the reaction vessel in a constant temperature bath maintained at 30° C; and (3) immersing the electrodes to a predetermined depth in the stirred pulp and imposing the desired current load to give a current density of 0.5 amperes per square inch. Figure 3 shows a typical electrode assembly used for electrolysis. Pulp samples were taken periodically and filtered. Both the filtrate and solids were analyzed for silver and mercury. At the end of oxidation time, the electrodes were removed, and the pulp was stirred overnight (16 hours) to allow for reaction of the residual sodium hypochlorite.

Cyanidation experiments were conducted in bottles placed on rolls. Cyanide ion concentration was determined by titration with silver nitrate using 5 (p-Dime thylamineobenzylidene) -rhodanine (SCSNHCOC: CHC6H4N (CH3)2).
Solution samples were analyzed for silver and mercury by an atomic absorption method. Silver in solid samples was analyzed by fire assay.
Mercury contained in solid samples was analyzed with a J-W/Lemaire mercury sniffer. Sodium hypochlorite was determined by KI-thiosulfate method, and chloride ion concentration was determined by titration with silver nitrate, using potassium dichromate as an indicator.
Results and Discussion
Electrooxidation of Unionville Tailings
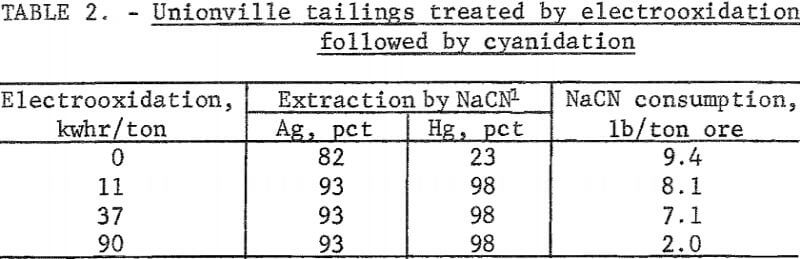
Silver tailings from Unionville, Nev, containing 8.0 ounces silver per ton and 4.4 pounds mercury per ton were treated by conventional cyanidation techniques. Silver extractions ranged between 65 and 82 percent, and corresponding mercury extractions were typically in the 20-percent range. Cyanide consumption was very high due to the copper remaining from the Washoe process. A series of experiments was conducted using electrooxidation of tailings pulped with 10 percent brine as a pretreatment to oxidize any cyanicides such as copper that were present in the tailings. The pulp was cyanided after the 10-percent sodium chloride electrolyte containing a portion of the copper had been removed by filtration. Table 2 shows that silver and mercury extractions increased to 93 and 98 percent, respectively, using a power consumption of only 11 kilowatt-hours per ton of ore. Corresponding sodium cyanide consumption amounted to 8.1 pounds per ton of ore. Increasing the electrooxidation time did not increase metal extraction, but the cyanide consumption was reduced to 2 pounds per ton of ore.
A second series of experiments to determine the effect of power input on extraction of silver established that 93 percent of the silver and 98 percent of the mercury could be extracted as a soluble tetrachloro complex without subsequent cyanidation. The extractions leveled off at 37 kilowatt-hours per ton and were not increased by further treatment.
The data indicate that the oxidizing system liberates an additional 11 percent of the silver (82 percent extraction by standard cyanidation technique) and that the liberated silver is soluble in a suitable complexing agent such as a chloride or cyanide. Also, the oxidizing system destroys cyanicides. Cyanide consumption decreased from 9.4 to 2.0 pounds per ton after electrooxidation treatment using a power consumption of 90 kilowatt-hours per ton of ore
Electrooxidation of Austin Tailings
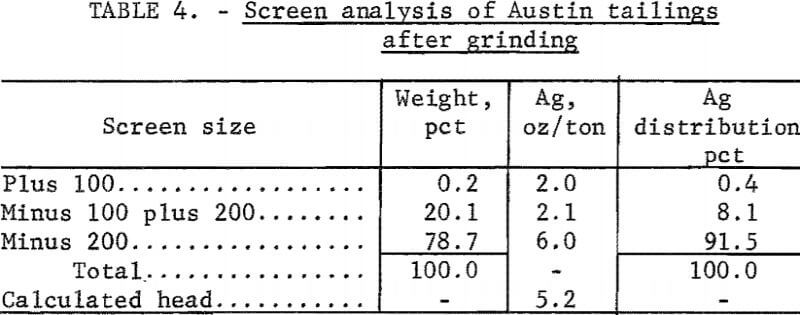
Tailings from Austin, Nev, which had been treated originally by salt roasting followed by amalgamation, were screened on a 20-mesh screen. The plus 20-mesh material was discarded because it contained only 0.1 ounce of silver per ton of ore. The minus 20-mesh fraction was then ground to minus 65 mesh; the resulting size distribution is shown in table 3. Cyanidation of these tailings, which contained 5.1 ounces silver per ton and 2 pounds of mercury per ton, resulted in extractions of 46 percent of the silver and a negligible amount of the mercury. Cyanide consumption was 3.3 pounds per ton of ore. Electrooxidation of 4 pounds of tailings in 20 percent brine yielded 90 percent mercury and 65 percent silver extraction using a power consumption of 50.2 kilowatt-hours per ton. Increasing the electrolysis time increased mercury extraction to 95 percent, but did not affect silver extraction. Cyanidation of the electrooxidized tails did not increase silver extraction.
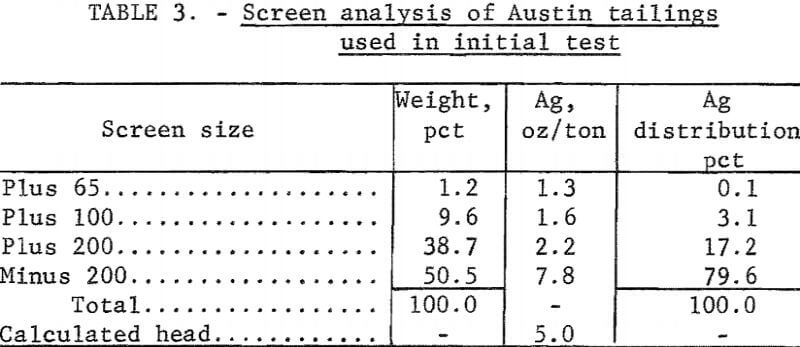
The effect of grinding on silver extraction was investigated to determine if the remaining silver was locked in the siliceous gangue. Minus 20-mesh tailings were ground to essentially 100 percent minus 100 mesh (table 4). Electrooxidation of this material in a 20-percent sodium chloride solution resulted in 77 percent silver extraction, and corresponding mercury extraction was 95 percent. Power consumption was 50.2 kilowatt-hours per ton. Neither extension of the electrolysis time nor regrinding of the tailings was beneficial in increasing the recovery of the metals. The treated tails were studied mineralogically, and it was concluded that the majority of the silver mineral remaining in the tails was similar to argentojarosite, which had been altered to some extent by the Reese River process.
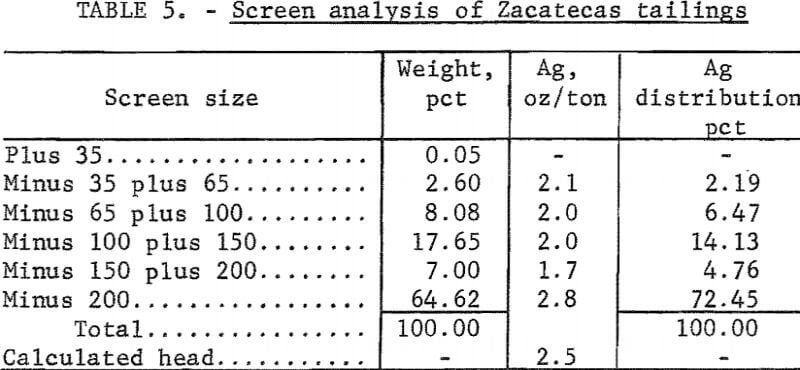
Electrooxidation of Zacatecas Tailings
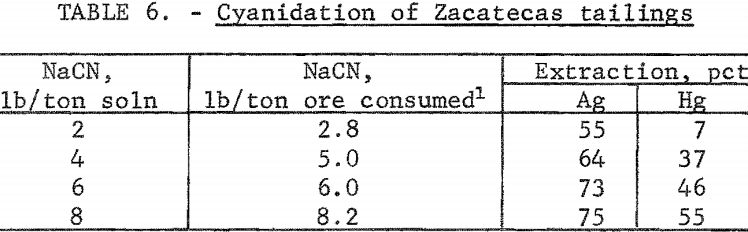
The tailings from Zacatecas, Mexico, were from old Spanish workings dating back to the 16th century. The Patio process had been used originally to extract the silver. The tailings had been washed several miles from the original mill by rain and had collected in a natural basin. The head analysis was as follows: Silver, 2.6 ounces per ton; mercury, 1.3 pounds per ton; gold, 0.013 ounce per ton; copper, 0.13 percent; zinc, 0.4 percent; lead, 1 percent; total carbon, 1.3 percent; and organic carbon, 0.70 percent. The screen analysis in table 5 shows the silver is disseminated throughout all screen fractions; the minus 200-mesh material contained the majority of the silver values. The tailings were cyanided at 40-percent pulp density using 2, 4, 6, and 8 pounds of cyanide per ton of solution. The data in table 6 show that silver extraction increased from 55 to 75 percent by increasing the addition of sodium cyanide; mercury extraction increased from 7 to 55 percent. The high cyanide consumption was attributed to the presence of cyanide-soluble copper and zinc in the tailings. The feasibility of economically cyaniding this ore is questionable because of the cyanide consumption and low mercury recovery.
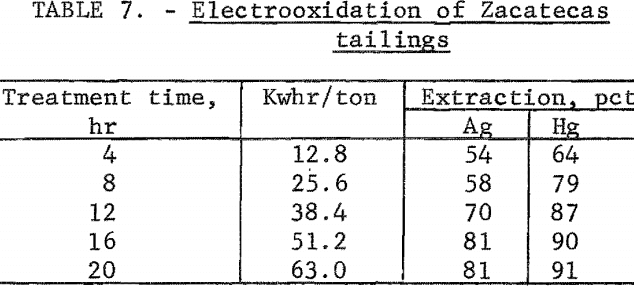
A series of experiments was conducted by treating 4-pound samples of tailings using a 20-percent sodium chloride solution and a 2 ampere-hour electrolysis rate. The data in table 7 show that silver and mercury extraction increased with electrolysis time and leveled off after 16 hours of treatment. Power consumption was 51.2 kilowatt-hours per ton of ore. Finer grinding of the tailings had essentially no effect on silver extraction.
Recovery of Silver and Mercury From the Pregnant Solutions
Silver and mercury were precipitated from the pregnant electrooxidized solutions using iron powder (90 percent iron by weight). Silver and mercury precipitations of 99.6 and 99.9 percent, respectively, were obtained from the pregnant solutions using 2 pounds of iron per pound of mercury and silver contained in the pregnant solution. The pH of these solutions ranged from 7.0 to 7.8.
Heating the precipitate to 550°-600° C and condensing the mercury vapors resulted in a 98-to 99-percent mercury recovery. Silver was recovered from the mercury calcine by conventional fire-refining techniques.
Conclusions
Electrooxidation of silver tailings from three different deposits was shown to improve silver extraction from 6 to 25 percent over cyanidation treatment. Silver extraction ranged from 77 to 93 percent, depending on the tailings; concomitant mercury extraction ranged from 90 to 95 percent. The amount of power required for the electrooxidation was in the range of 51.7 to 90 kilowatt-hours per ton of tailings. The data indicate that the electrooxidation technique may have economic potential for recovery of silver and mercury from tailings.
