Classic flotation and roasting procedures for recovering molybdenum oxide from molybdenum source materials frequently result in low recovery of molybdenum. This is particularly true in molybdenum flotation from porphyry copper ores where difficulties can he encountered in refloating molybdenite which has been recycled to rougher and cleaner flotation sequences. Because copper-molybdenum separation may be less than desired, a portion of the molybdenum may be lost when the material is charged to the reverberatory furnace to recover associated copper. Rhenium is intimately associated with molybdenite. Consequently, not only do rhenium losses closely parallel molybdenum losses in recovery by flotation, but additional losses are encountered in scrubbing the rhenium from off-gases obtained during the roasting of molybdenite to molybdic oxide. Finally, roasting of the molybdenite product to obtain molybdic oxide results in formation of sulfur oxide gases which arc discharged to the atmosphere as pollutants.
Improved molybdenum recovery from ores and concentrates has been shown to be feasible without SO2 emission by using a sodium hypochlorite leach system for dissolving molybdenum as the molybdate ion. The stoichiometry for the reactions is as follows:
MoS2 + 9OCl- + 6OH- → MoO4= + 9Cl- + 2SO4= + 3H2O…………………………………..(1)
The technique is reported to be effective for dissolving molybdenum, but the high cost of reagents consumed in the reaction makes the process questionable for commercial use.
The Bureau of Mines has developed an electrooxidation technique for treating carbonaceous gold ores and for recovering mercury from cinnabar ores which consists of generating hypochlorite in situ in a brine-ore pulp by electrolysis. The chemistry of producing hypochlorite in the brine-ore pulp is as follows:
Anode 2Cl- → Cl2 + 2e-………………………………………………………………..(2)
Cathode 2H2O + 2e → 2OH- + H2………………………………………………(3)
The chlorine and hydroxyl ion produced then combine to form the hypochlorite ion,
2OH- + Cl2 → OCl- + H2O + Cl-…………………………………………………(4)
The power required to produce 1 pound of sodium hypochlorite in the ore pulp by electrolysis is in the range of 1.5 to 2 kwhr, indicating that the system might possess potential as an economical oxidation-leaching technique for extracting molybdenum and rhenium from their source materials. Molybdenum and rhenium values would be recovered from pregnant solutions by known solvent extraction techniques. This paper discusses results obtained by using the electrooxidation technique on molybdenum ore.
Procedure and Apparatus
Laboratory electrooxidation experiments were conducted on an MoS2 ore obtained from Colorado. Partial analysis of the ore, in weight-percent, was as follows: Molybdenite, 0.35; copper, 0.01; iron, 0.71; sulfur, 0.75; and trace amounts of lead and zinc. Mineralogical examination of the ore indicated that major components of the rock were quartz and potassium feldspar with minor amounts of fluorite, calcite, molybdenite, sericite, and muscovite. Occasional occurrences of apatite and rutile were also noted.
Laboratory-scale experiments were conducted on 908-gram samples of the ore ground to the desired screen size and pulped together with 200 grams of NaCl, 13.7 grams of Na2CO3, and 2,000 ml of water in a 4-liter beaker. Electrodes were immersed in the pulp to the depth required to give the
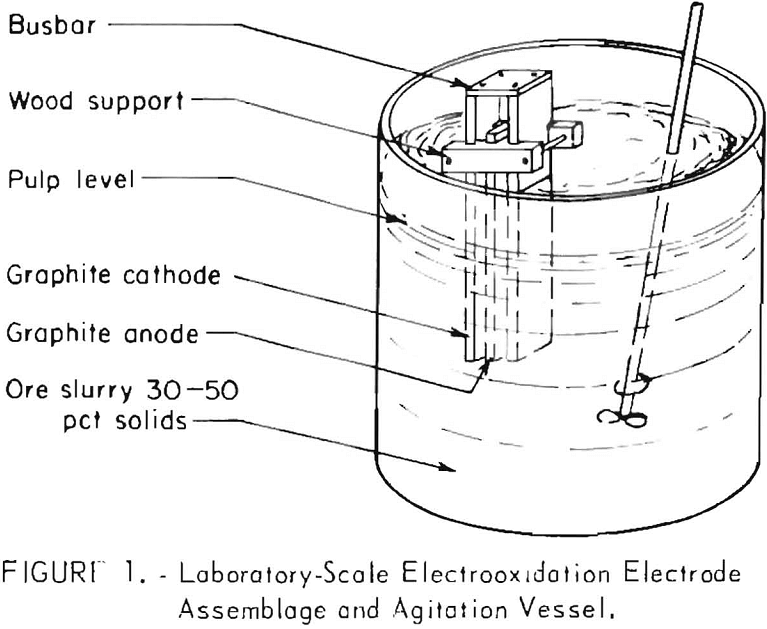
desired current density; then direct current was applied to the system at a selected rate. The temperature was held constant by placing the apparatus in a water bath. Figure 1 shows the electrode assembly suspended in a beaker of pulp.
Experiments using technical-grade MoS2, (96 percent pure) were conducted in the following manner: MoS2 (6.7 grams) was pulped with 2,000 ml of water containing 200 grams of sodium chloride. A current of 7.5 amperes at a current density of 0.5 amp/in² was applied for 5 hours. Sodium carbonate was added to maintain a pH range of 6.5 to 7.5. During the first 3 hours, 16.5 grams of Na2CO3 was added. The pH dropped from 7.5 to 7.1 during the last 2 hours of oxidation with no additional Na2CO3. The slurry was filtered, and the residue and filtrate were analyzed for molybdenum.
Analysis for molybdenum and rhenium in solution was accomplished by atomic absorption techniques. Molybdenum in solid samples was analyzed by chemical and X-ray diffraction techniques. Rhenium in solid samples was analyzed by an extraction-atomic absorption technique.
Sodium hypochlorite and sodium chlorate concentrations in leach solutions were determined by the KI-thiosulfate method (iodometric method). Sodium chloride concentration was determined by titration with silver nitrate, using potassium dichromate as an indicator. Carbon, nitrogen, and sulfur were determined by standard combustion techniques.
Results and Discussion
A series of preliminary experiments was conducted electrooxidizing samples of MoS2 and specimen molybdenite to determine the amenability of the mineral to treatment by the electrolytic procedure. Constant operating conditions of 30° C, 10 percent salt concentration, and 5 hours of electrolysis at 7.5 amperes for the experiments were based largely on experience gained in treating gold, silver, and mercury ores. The data indicated that molybdenum extraction into solution as the molybdate ion did not start until electrolysis had been conducted for several minutes. After this initial conditioning period, the extraction was almost linear with respect to electrolysis time. No attempt was made to optimize the reaction because the concentrates and ores planned for subsequent investigation contained other sulfide minerals.
However, the results were favorable in that it was shown that molybdenite is amenable to electrooxidation treatment in the range of pH 6 to 8.
Experiments electrooxidizing ore that contained 0.35 percent MoS2 were conducted to determine the effects of salt concentration, particle size of the ore, temperature, current density, pH, and treatment rate.
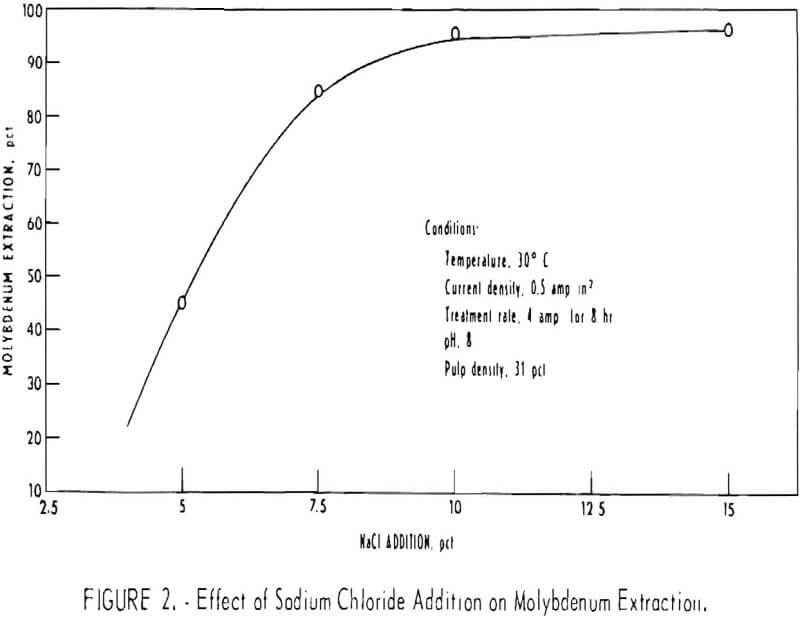
The effect of salt concentration on molybdenum extraction was determined in a series of experiments varying the salt concentration of the electrolyte between 5 and 15 weight-percent. Constant operating conditions of 30° C, 0.5 amp/in² current density, and a treatment rate of 4 amperes at 3.5 volts for 8 hours were used for the experiments. Sodium carbonate was used as the source of the hydrooxyl ion. The final pH was in the range of 7 to 8. The data in figure 2 show that molybdenum extraction increases rapidly to 85 percent on increasing salt concentration from 5 to 7.5 percent. The extraction levels off at 95.2 percent as the salt concentration is increased from 10 to 15 percent. The increase in production of hypochlorite with an increase in salt concentration in the electrolyte is attributed to the resulting higher concentration of chloride ion at the surface of the anode. Under these conditions, the higher chloride concentration inhibits the formation of oxygen by the electrolysis of water.
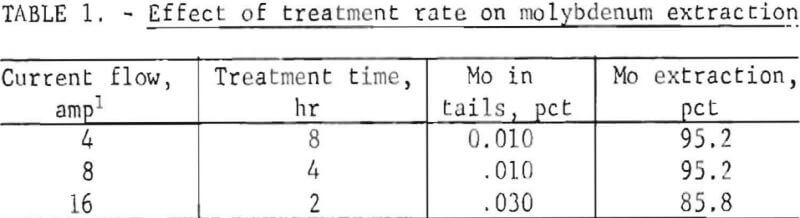
The rate of treatment (rate of current flow) of the ore is an important variable because it determines the rate of hypochlorite production and consequently the concentration of hypochlorite in the cell. Table 1 summarizes molybdenum extraction data obtained under constant conditions of 10-percent salt concentration, 30° C, and 0.5 amp/in² current density. Molybdenum extraction remained constant at 95.2 percent when the current flow was increased from 4 to 8 amperes, and then dropped sharply to 85.8 percent when current flow was increased to 16 amperes. The reaction of hypochlorite at the anode to
form the chlorate ion is known to occur, presumably under conditions of high treatment rates (high current flow); the concentration of hypochlorite in the electrolyte becomes sufficiently high to result in formation of chlorates.
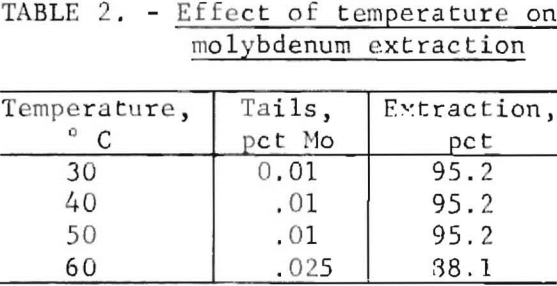
The effect of temperature on molybdenum extraction was determined at 30°, 40°, 50°, and 60° C. Table 2 shows that temperature had little effect upon extraction until 60° C was reached. At this temperature, extraction decreased from 95.2 to 88.1 percent. The decline in molybdenum extraction with increasing temperature is attributed to the increased rate of production of chlorate by the chemical reaction
3OCl- → ClO3- + 2Cl-…………………………………………….(5)
The chlorate ion in this system does not act as an oxidant at a neutral pH. The temperature of the pulp can be controlled easily by a variety of parameters such as salt concentration, pulp density, current density, treatment rate, size and shape of agitation tanks, and electrode spacing. However, when a concentrate is treated, so much power is required that cooling will be necessary.
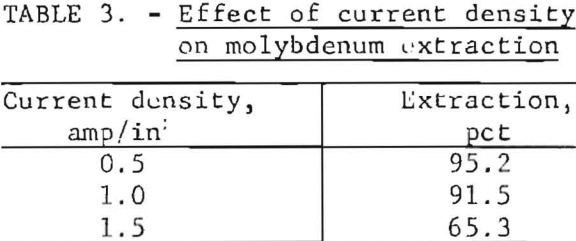
Current density is a critical parameter because excessive currunt densities tend to increase the temperature and decrease the efficiency of the desired chemical reactions at the electrode-electrolyte interface. Three experiments were conducted using current densities of 0.5, 1.0, and 1.5 amp/in² with the results shown in table 3. The data show that molybdenum extraction decreased 30 percent when the current density was increased from 0.5 to 1.5 amp/in². A current density of 0.5 amp/in² or less is recommended; however, the tonnage of a mill and the agitator size required are important considerations in projecting the practical number of electrodes to correlate with the consequent current density that must be used.
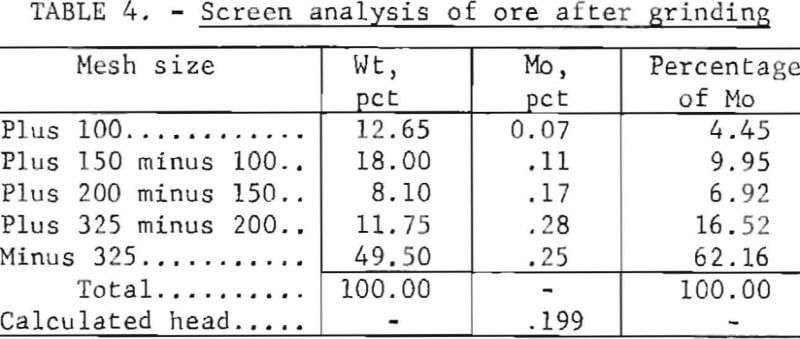
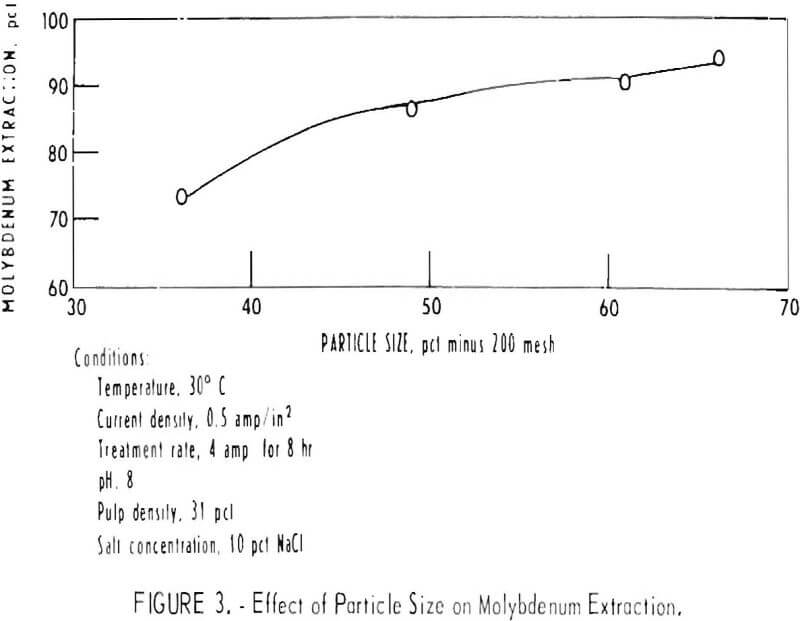
The particle size of the ore to be treated is an important part of any hydrometallurgical process, because the mineral must be liberated from the gangue material. The effect of particle size on molybdenum extraction was investigated using four different grinds. The distribution of molybdenum in the various size fractions for one particular grind is shown in the screen analysis in table 4. The other three grinds used have a similar overall molybdenum distribution. The relationship between the amount of minus 200-mesh particles present in the four different grinds and the degree of molybdenum extraction is shown in figure 3. Molybdenum extraction increases almost linearly from 73 percent with a grind containing 36.2 percent minus 200 mesh to 95.2 percent with a grind containing 66.3 percent minus 200 mesh.
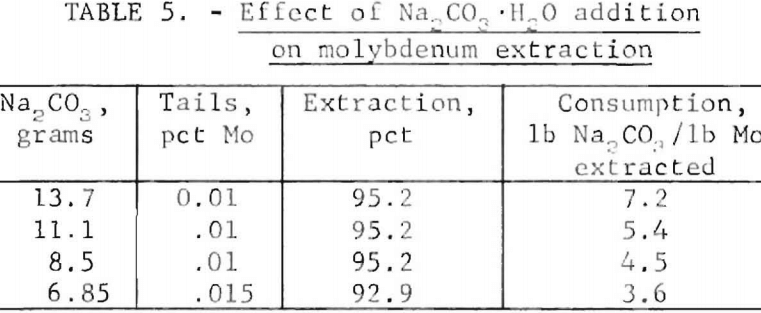
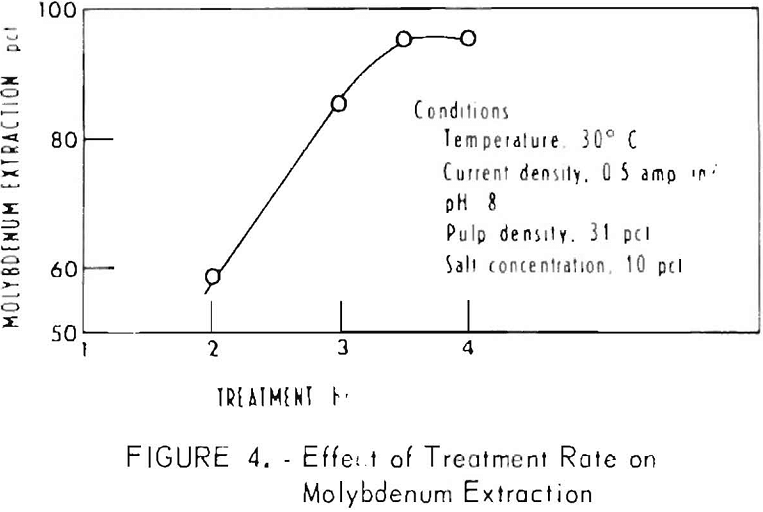
In the oxidation of molybdenite by hypochlorite to form the soluble molybdate ion, the hydroxyl ion is needed, as shown in equation 1. Accordingly, the pH of the electrolyte would be expected to be an important parameter. The effect of pH on the system, as measured by the amount of Na2CO3, added, was determined in a series of experiments adding 6.85 to 13.7 grams of Na2CO3 to 908 grams of the ore. The data in table 5 indicate that optimum extraction occurs when 4,5 to 7.2 pounds of Na2CO3 per pound of extracted molybdenum is used. An experiment was conducted using operating conditions that were determined to be near- optimum in previous experiments consisting of the following: 30°C, 10 percent NaCl, current density of 0.5 amp/in², treatment rate of 8 amperes at 3.5 volts for 4 hours for a 908-gram sample, and 10 grams Na2CO3. Samples were taken at 2, 3, 3½, and 4 hours. Each sample was rolled overnight and then analyzed for molybdenum. Figure 4 shown the percent extraction of molybdenum versus hours of treatment. Maximum extraction was reached between 3 and 3½ hours of treatment. The final pH was 7.2, indicating that almost all of the Na2CO3 present was consumed. The power consumption is 24 kwhr per pound of molybdenum extracted.
Studies are in progress extending the electrooxidation treatment to low-grade molybdenite concentrates containing 4.5 to 30 percent MoS2 and 180 to 1,000 ppm Re. Laboratory-scale experiments electrolyzing 0.5- to 2-pound samples of molybdenum source materials in 10-percent brine solution with pulp densities in the 10- to 35-percent range all resulted in a tailing or gangue that contained 0.01 to 0.10 percent molybdenum, which corresponds to extractions of 90 to 99 percent molybdenum. Concomitant rhenium extraction was 95 to 99 percent. Power consumption was in the range of 16 to 24 kwhr per pound of molybdenum extracted. The optimum pH for oxidation
appears to be in the range of pH 6 to 8 with a temperature of 30° to 40° C. During the oxidation, the contained copper, which is mostly in the form of chalcopyrite, is not dissolved. Slight oxidation of the surface of the mineral does take place. Recovery of the molybdate and perrhenate ions from solution is

accomplished by conventional resin or liquid ion exchange methods using quaternary ammonium or tertiary amine compounds. Molybdenum-rhenium separation is effected by pH control and differential stripping or by a two-stage extraction sequence.
Pilot plant experiments are being conducted in the installation shown in figure 5 to obtain power and salt consumption data and to determine the effect of continuous recycle of brine solution on buildup of impurities in solution. Data from initial experiments closely parallel laboratory-scale data for power and salt consumption.
Conclusions
The Bureau of Mines has investigated an electrooxidation technique for extracting molybdenum and rhenium from ores and concentrates. Extractions of 90 to 99 percent molybdenum and 95 to 99 percent rhenium were obtained. Molybdenum and rhenium can be selectively dissolved from concentrates containing copper minerals. The power consumption was in the range of 16 to 24 kwhr per pound of molybdenum extracted. Pilot plant studies are now underway to evaluate the process on a larger scale.
