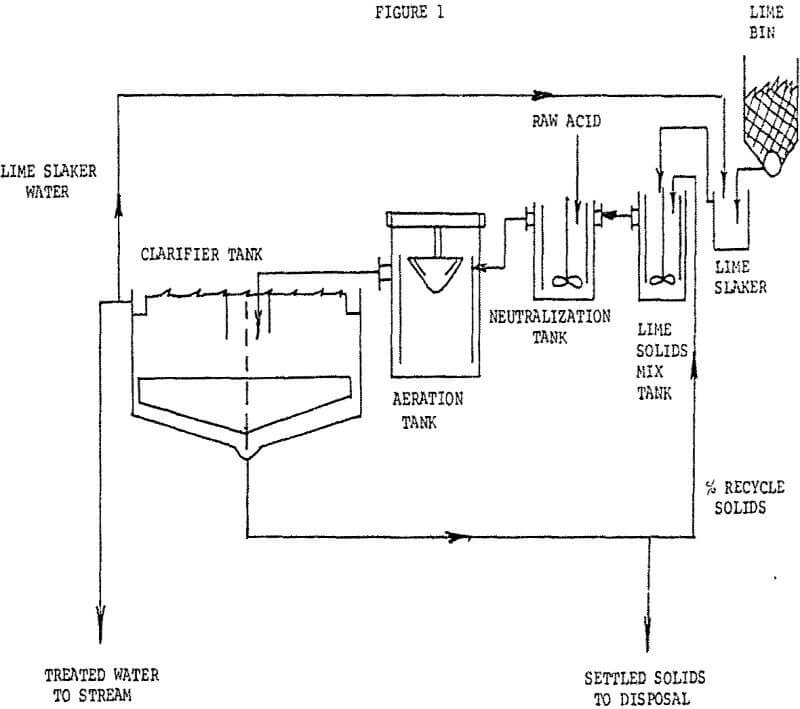
Because of increased pressure from Federal & State Regulatory-Agencies, most acid flows will require some sort of treatment prior to being discharged to receiving streams. In many industries, the volumes of liquors are large and their acid content varied.
“Standard” methods of acid neutralization with lime have been known for years, however, the volume and characteristics of the resultant precipitated solids can create a disposal problem. Also, when standard lime treatment is used, the settling characteristic of the precipitated solids is normally slow. In some instances, the necessity of added dissolved solids removal, (such as iron, needing aeration) add to the problem of concentrating neutralized solids.
The treatment consists of neutralization with a milk of lime solution followed by aeration to convert precipitated ferrous hydroxide to the ferric state, to reduce iron solubility, followed by sedimentation.
Clarifier overflow goes directly to a receiving stream. A percentage of settled solids from the clarifier are recycled to a lime reactor ahead of the neutralization tank. The remaining settled solids are pumped underground into an abandoned mine area.
This plant required a single 25-HP surface aerator, reaction tanks (with mixers), an 80′ diameter clarifier and a lime feeding system. Present lime usage is approximately 4 to 5 TPD of quick lime having 85 to 90% CaO availability. One man works the day shift with remaining two shifts per day run on automatic control based upon outfall liquor pH..
Spent acid liquors from a copper operation have been treated with lime and discharged into a receiving stream for many years. This disposal method complied with state standards until recently.
The neutralized liquors contain from 2 to up to 22 grams per liter of iron. As the discharged liquor flowed down a stream bed, considerable amounts of iron were precipitated out as evidenced by the color along the stream bank.
Preliminary laboratory tests showed that up to 7 minutes mixing time was needed for proper reaction between milk of lime solution and spent acid. For required reduction of iron and zinc content, a pH of 8 was necessary. If air were added during neutralization, a pH of 7 was adequate and the ferrous hydroxide was oxidized to a less soluble, more settlable, ferric hydroxide.
For each waste flow, we suggest that individual tests be made. A representative waste sample should be obtained and analyzed for pH, dissolved solids content, suspended solids and other conditions which may make it unsuitable for direct discharge.
Mix a milk of lime slurry (using lime similar to that available at proposed plantsite) with raw acid flow to reach desired pH and determine the volume (by weight) of precipitated solids. Multiple this weight (less weight of lime) by 20 to 25 times. Mix this amount of recycled solids with a milk slurry. Add this mixture to raw acid flow and note results. It may require a higher or lower recycled solids ratio but lab tests will determine this.