Table of Contents
The present process refers to a method for efficiently obtaining high purity metallic copper from copper ammonium sulfite. Essentially, this process consists of submitting the copper ammonium sulfite to the action of heat and pressure with the object of obtaining by the decomposition of it, metallic copper, ammonium sulfate, sulfur dioxide, and sulfuric acid.
Detailed Description of the Process
a). This process consists of a procedure to obtain, with high efficiency, pure copper beginning with ammonium copper sulfite and consists of the following steps :
- Preparation of an ammonical solution of copper in which this element is present as copper tetrammine sulfate of copper tetrammine carbonate ( Cu (NH3)4SO4 or Cu ( NH3)4CO3), the pH of which is between 9.0 and 10.5
- Treating the previous solution with sulfur dioxide to effect the precipitation of copper ammonium sulfite (NH4) CuSO3 ) and separation of this precipitate from the resulting solution of ammonium sulfate.
- Treating the copper ammonium sulfite obtained in the previous step in closed containers with heat and pressure to effect efficient decomposition of this compound to metallic copper, ammonium sulfate, sulfuric acid, and sulfur dioxide.
- Separation and washing of the metallic copper and preparation for market.
- Finally, recuperation of the ammonium sulfate, sulfuric acid, and sulfur dioxide produced in the decomposition of the copper ammonium sulfite.
The step ( 1 ) of the previously described items is an operation which is very well known by experts of the art, and its practical execution depends a great deal on the raw material available.
The precipitation of copper ammonium sulfite from this solution, can be expressed according to the following equation :
2Cu (NH3) 4 SO4 + 3SO2 + 4H2O → 2 (NH4) CuSO3 + 3 (NH4) 2 SO4
Furthermore, excess ammonia in the solution also reacts with additional sulfur dioxide to produce ammonium sulfate according to the following reaction :
2NH4OH + SO2 + ½ O2 → (NH4)2 SO4 + H2O
Step ( 3 ) is the essential part of our process and consists of the thermal decomposition of copper ammonium sulfite to produce metallic copper, ammonium sulfate, sulfuric acid, sulfur dioxide, and only minimum quantities of copper sulfate.
The quality of the copper ammonium sulfite, in terms of both chemical and physical characteristics, has a significant influence on the efficiency of thermal decomposition to metallic copper. In addition to those characteristics already mentioned, we have found that high efficiency can be improved when the sulfite has been dried at a temperature not to exceed 120 °Centigrade.
The chemical composition of the solution in which the thermal decomposition of copper ammonium sulfite takes place is extremely important from the standpoint of efficiency. Although reaction takes place when pure water is used, the highest efficiencies are obtained using a solution containing sulfuric acid and ammonium sulfate such as that obtained in previous reactions which has become nearly saturated with ammonium sulfate.
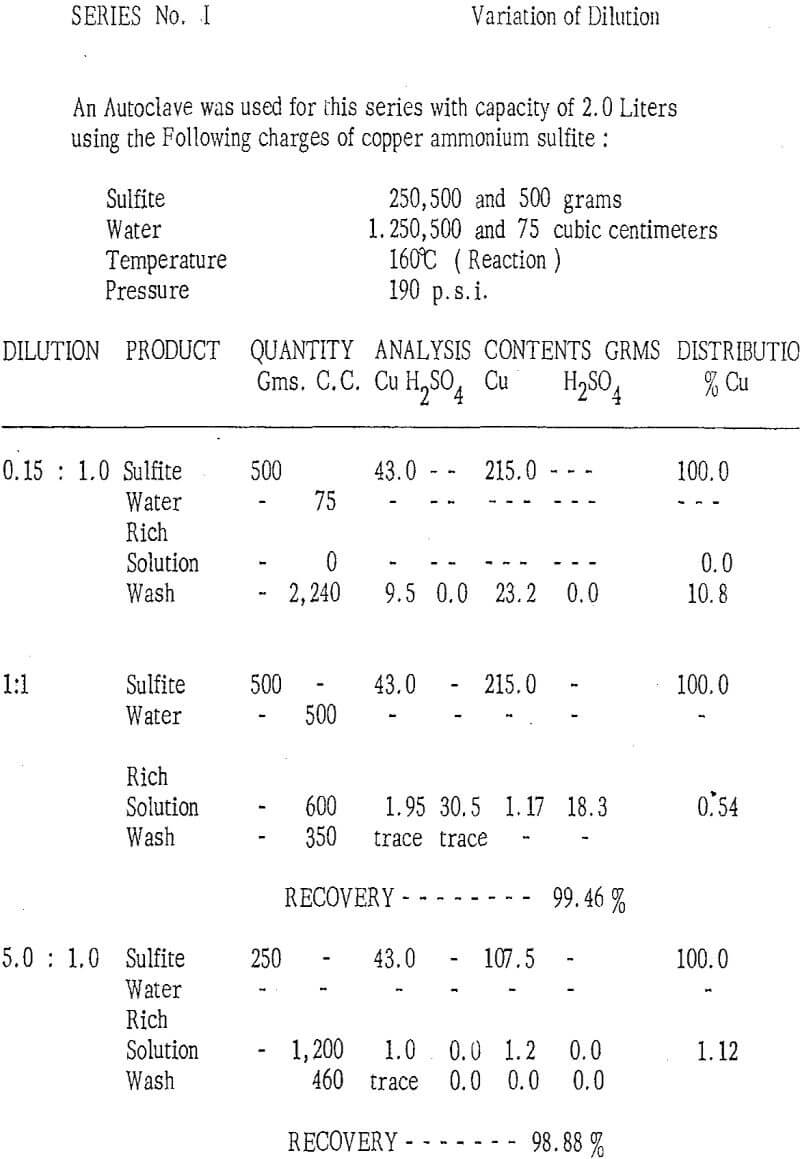
Under appropriate conditions, the decomposition reaction generates a large quantity of sulfur dioxide, which results in an increase of pressure within the autoclave. It has been observed that limited control of pressure is important to the quality of the copper product as well as to the efficiency of its production. If the pressure does not reach a minimum limit, reaction is incomplete while, on the other hand, if the pressure exceeds a maximum limit, part of the copper reacts to form a mixture of copper sulfate and copper oxide. It has been found experimentally, that the optimum pressure for the decomposition of copper ammonium sulfite lies in the range from 10 to 15 kilograms per square centimeter (i.e., 150 to 220 psi. )
Examples of the Process
As described above, various amounts of copper ammonium sulfite were obtained from solutions of copper tetrammine sulfate ( Cu (NH3)4SO4) at pH 10. 0 X – ray diffraction analysis, showed the products to be homogeneous, well – crystallized ( NH4 ) CuSO3 Microscopic examination revealed the crystals to be colorless, transparent hexagonal plates with average dimensions of 200 microns in diameter by 25 microns thick. The copper content of the sulfite, was dound to be 43 %. The sulfite was separated from the original solution by filtration and was dried to 3.0 % moisture, at a temperature of 120 ° Centigrade without washing. This product was used in the various tests described below :