Table of Contents
This investigation was conducted by the Bureau of Mines in support of its overall goals of helping to maintain an adequate supply of minerals and metals for future national needs with minimum waste and environmental degradation. The objective of the investigation was to develop a hydrometallurgical method of producing zinc from ZnS (sphalerite) concentrates without the attendant formation of (sulfur dioxide) , which requires the use of air pollution controls. Experimental conditions were sought to produce elemental sulfur at atmospheric pressure concurrent with a high recovery of zinc.
Currently, zinc is commercially produced by roasting ZnS concentrates in air to produce ZnO and SO2 . ZnO in the calcine is leached with recycled electrolyte containing dilute H2SO4 (sulfuric acid). Various purification processes are used to recover cadmium and silver and to remove deleterious impurities before zinc is electrowon. The SO2 produced by roasting is converted to H2SO4 Supplies of H2SO4 have increased in recent years as a result of antipollution regulations that require control of SO2 emissions from industrial sources. Sulfuric acid may not be marketable in the geographical area where it is produced, and transportation costs diminish its value as a byproduct. If the sulfur could be recovered in elemental form, its market potential would be increased.
Over 60 pct of domestic primary demand for zinc is met by imports; the U.S. share of world zinc production has declined from 27 pct in 1960 to 9 pct in 1974. One way to help reverse this trend is to provide better technology for zinc production, especially technology that does not require costly pollution controls.
Direct leaching of metal sulfide concentrates with H2SO4 has been investigated. McKay and Halpern studied the oxidation of pyrite with oxygen in acid solutions and observed a two-stage reaction; in the first stage, H2S (hydrogen sulfide) and elemental sulfur were formed, and, in the second, SO2 and H2SO4. Other investigators determined the effect of many variables on the reaction and its products. Bjorling reported on similar experiments with synthetic ZnS and H2SO4; elemental sulfur was produced, but the reaction rate was very slow. Using natural ZnS, Forward was able to extract 95 pct of contained zinc in 4 hours at 115° C with an oxygen pressure of 20 lb/sq in. Sulfur formed solid pellets and occluded some of the ZnSO4. Stanczyk and Rampacek, in similar experiments, formed H2SO4 at 200° C. Other researchers have determined the reaction kinetics of ZnS dissolution in dilute H2SO4, which produces H2S.
Experimental Procedures
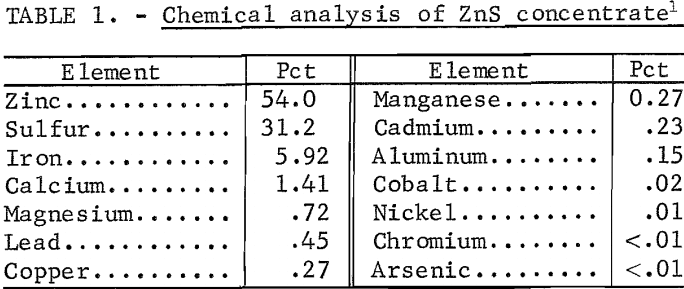
The feed for the tests was a typical Missouri ZnS concentrate. Chemical analysis and particle-size analysis of the head sample are given in tables 1 and 2, respectively. Calcium and magnesium occur in the concentrate, largely as carbonates. Concentrates containing such appreciable quantities of carbonates are usually preleached with dilute H2SO4 to remove Mg and CO2 before roasting; however, the concentrate used in the present investigation was not preleached.
A 1,000-ml batch reactor was used to react 200-gram samples of the ZnS concentrate with 250 ml of H2SO4 solution. The reactor was sealed from the atmosphere, and gases evolved by the reaction were collected and measured over water, with minimum contact area between gases and water. Analysis of the water revealed that the amount of dissolved gases was negligible. Samples of the evolved gases were removed with a small gas syringe through a septum in the reactor. The gas composition was determined by gas chromatography, using a Porapak Q column at 25° C to separate air, CO2, H2S, and SO2.
The ZnS samples were heated in the reactor at 130° C for 30 min to remove all moisture prior to testing. After adding the acid, the mixture was stirred and the temperature was gradually increased to the desired level. After reaction, the mixture was cooled, then filtered or centrifuged to recover moderately concentrated H2SO4. The solids then were leached with hot, dilute H2SO4 (simulated depleted electrolyte) or water to dissolve the ZnSO4. Filtration or centrifugation produced a residue consisting of elemental sulfur, insoluble portions of the concentrate, and unreacted ZnS. The filtrate was cooled, and ZnSO4·7H2O was crystallized out and filtered.
In other tests, reaction rates were determined by reacting 200 grams of ZnS with 250 ml of 83-pct H2SO4 at approximately constant temperature. Small samples of the reaction mixture were removed at intervals and quenched in water. The insoluble residue was filtered, dried, weighed, and analyzed for zinc.
Isothermal tests were made in a 1,000-ml flask maintained at constant temperature by an oil bath. Five-gram samples of ZnS were added through an air lock to the sealed flask, which contained 50 ml of H2SO4 solution and air. Gases in the flask were sampled and analyzed by chromatography, and the volume of sulfur gases under standard conditions was calculated.
Results and Discussion
Isothermal Tests
The desired reaction was to combine ZnS and H2SO4 to produce ZnSO4, S, and H2O. Isothermal tests were conducted at several temperatures and acid concentrations to determine the changes in the relative amounts of H2S and SO2 evolved during the reaction. An excess of acid was used, which kept the acid concentration almost constant during the reaction. Figures 1 and 2 show the quantities of gases evolved at various acid concentrations and at two temperatures. In all tests, some H2S formed initially and then reacted with the mixture to form sulfur. Sulfur dioxide evolved as the reaction progressed. The reaction is sensitive to changes in H2SO4 concentration; 75-pct H2SO4 produced large amounts of H2S (fig. 2), and 87.5-pct acid yielded SO2 primarily (fig. 1). In these tests using an excess of acid, the optimum acid concentration to minimize sulfur gas evolution was about 80 pct.
The evolution of H2S and SO2 was attributed to the following generalized reactions:
ZnS + H2SO4 → ZnSO4 + H2S……………………………………………………………………………(1)
and ZnS + 4H2SO4 → ZnSO4 + 4SO2 + 4H2O…………………………………………………….(2)
Figures 3 and 4 show the quantities of gases evolved at different temperatures and two acid concentrations. Increased temperatures resulted in increased amounts of SO2. In most tests, the amount of SO2 produced leveled off after a time; thereafter, little or none was produced. This suggests that the SO2 and the reaction mixture were nearly in equilibrium. Figures 1
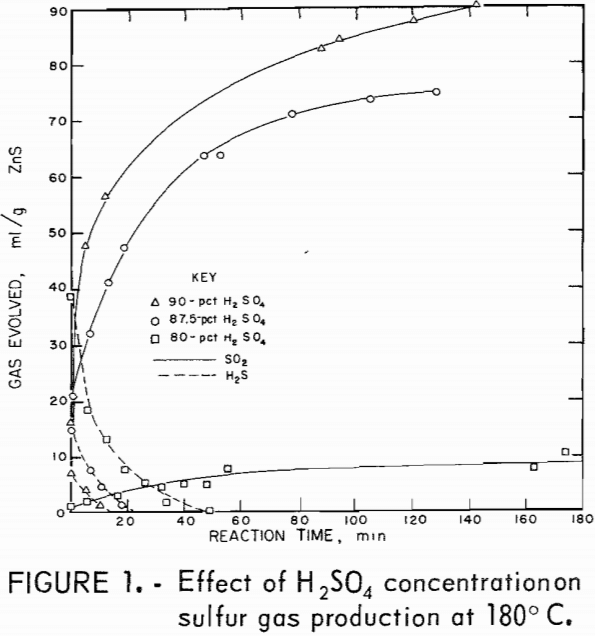
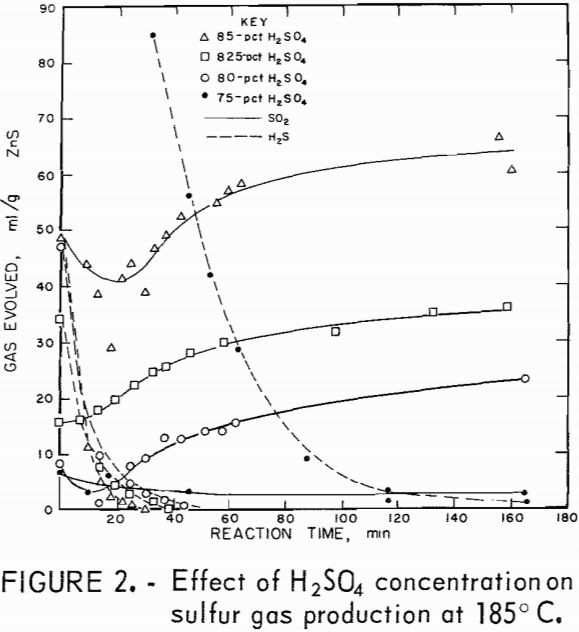
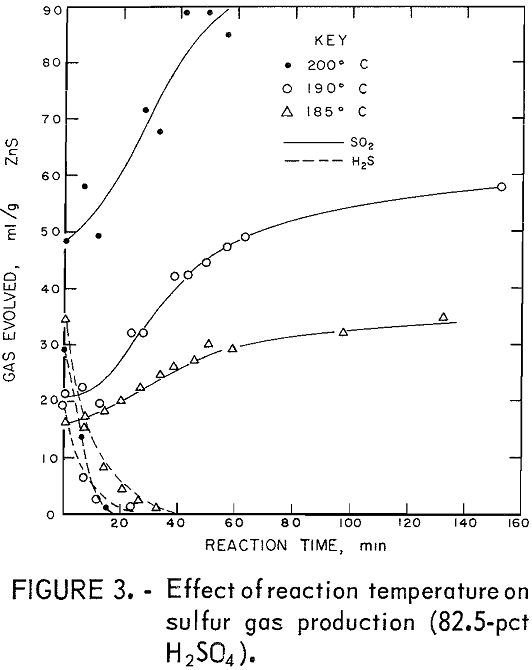
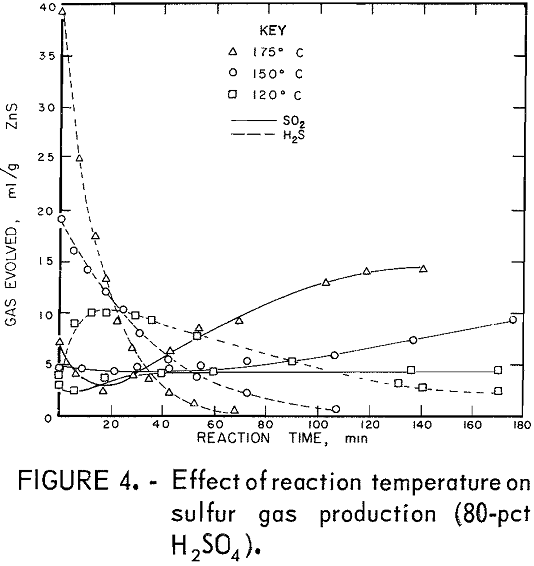
through 4 show that acid concentration is a more critical factor than temperature in determining whether H2S or SO2 is primarily produced.
Hydrogen sulfide was found to disappear from the system by a first-order reaction at 120° to 185° C. An Arrhenius activation energy of +13 kcal/mole for the reaction was determined from a plot (fig. 5) of the logarithms of the temperature-dependent rate constant as a function of reciprocal temperature (1/T). The rate of disappearance of H2S from the system is given by

where T is the temperature, R is the gas constant, and PH2s is the pressure of H2S.
The activation energy of +13 kcal/mole for the disappearance of H2S indicates that the reaction was not diffusion controlled. (Diffusion controlled reactions have activation energies on the order of 1 kcal/mole.) The H2S probably reacted with SO2 in the vapor phase.
The ZnS concentrate contained some calcium and magnesium carbonates, which, when contacted with acid, release CO2. The large initial evolution of H2S is probably because the CO2 sweeps the H2S out of the reaction mixture.
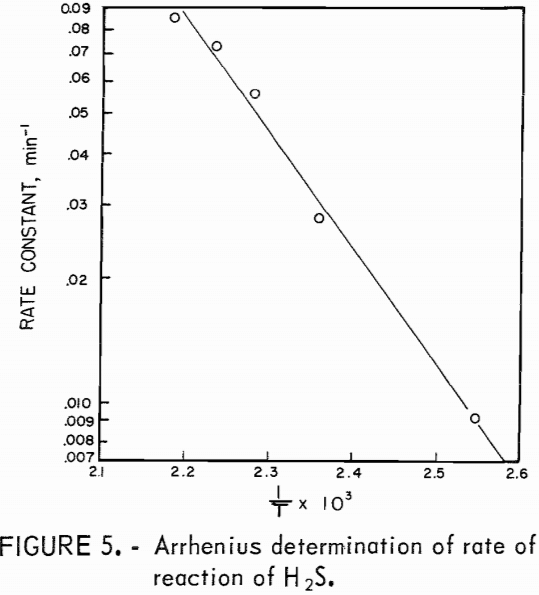
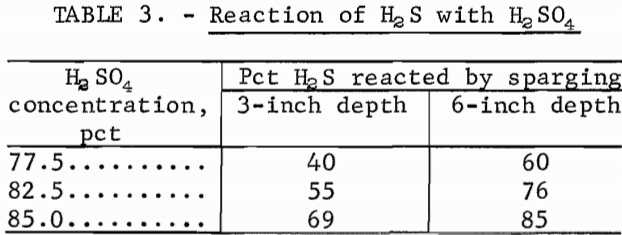
Table 3 shows the results of tests in which H2S was bubbled through H2SO4 solutions from two different depths. The acid temperature was 180° C, and the H2S flow rate was 100 ml/min. The percentage of H2S that reacted to form elemental sulfur increased as the depth traversed increased, and as the acid concentration increased. If the ZnS were introduced at the bottom of the reaction vessel, the amount of H2S produced would be less as the depth of acid was increased.
Reaction Stoichiometry
Table 4 gives the results of batch tests in which far less excess acid was used and conditions were near optimum for the formation of elemental sulfur. Initial acid concentrations ranged from 83 to 85 pct, and only small amounts of sulfur gases were produced. The average yield of sulfur was 1.33 moles per mole of ZnS reacted. Stoichiometry of the reaction is given by
3ZnS + 4H2SO4 → 4S° + 3ZnSO4 + 4H2O………………………………………(4)
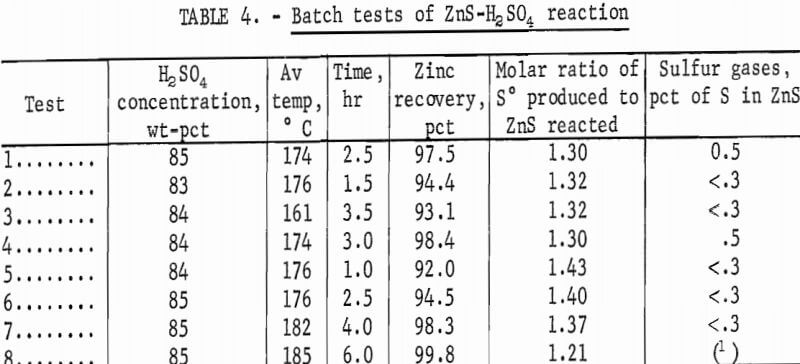
The effect of reaction temperature on stoichiometry was negligible. Higher temperatures accelerated the reaction and the consequent rate of heat evolution from the moderately exothermic reaction. With long reaction times, as in tests 7 and 8 (table 4), zinc recoveries approached 100 pct. High temperature and long reaction time (test 8) resulted in decreased sulfur recovery; this was attributed to the oxidation of elemental sulfur by H2SO4, as follows:
S° + 2H2SO4 → 3SO2 + 2H2O………………………………………………………….(5)
The formation of SO2 by reaction of ZnS and H2SO4 cannot be supported because of the near absence of unreacted ZnS after the first 2 hours of reaction.
Rate of Reaction
A series of leaching tests were conducted in which the reaction time and the temperature were varied. The concentration of unreacted ZnS was calculated from the weight and analysis of the leach residue using an empirically derived equation, and the logarithms of these values were plotted as a function of time (fig 6). The approximate linearity of the plots indicates that the reactions were approximately first order, depending on ZnS concentration. The rate constants were determined from the slopes. The extrapolated lines do not go through one point because different times were required to stabilize the temperatures at the desired values. The finely ground ZnS reacting with an excess of acid solution is a pseudohomogeneous mixture. The integrated rate equation for a first-order reaction is
[ZnS] = [ZnS]t = 0 exp(-kTt)………………………………………………………(6)
where kT is the temperature-dependent rate constant and [ZnS] is the concentration of unreacted ZnS at time t.
The following Arrhenius equation was used to determine the effect of temperature on the reaction rate constant:
kT = Ao exp(-E°/RT)…………………………………………………….(7)
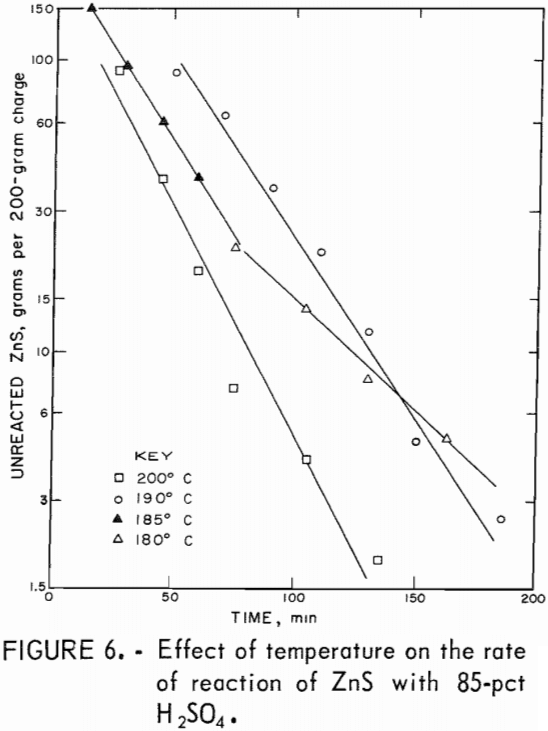
where Ao is the frequency factor and E° is the Arrhenius activation energy of the reaction. The values were calculated to be 4.2 x 10 6 min-¹ for the frequency factor and 17.4 kcal/mole for the activation energy. The reaction time needed to obtain 98.0 pct conversion of ZnS at 200° C was calculated to be 1.3 hours; at 180° C, 3.2 hours would be needed. These calculated values compare satisfactorily with experimental zinc recoveries given in table 4.
Separation of Reaction Products
The reacted mixture contained ZnSO4, H2SO4, S, and unreacted solids. In one approach, the insoluble residue was removed by leaching the soluble components with water and filtering. The filtrate contained the H2SO4 and ZnSO4. The acid must be separated from the ZnSO4 in order to use the electrolytic zinc process, which requires a neutral zinc sulfate solution for purification.
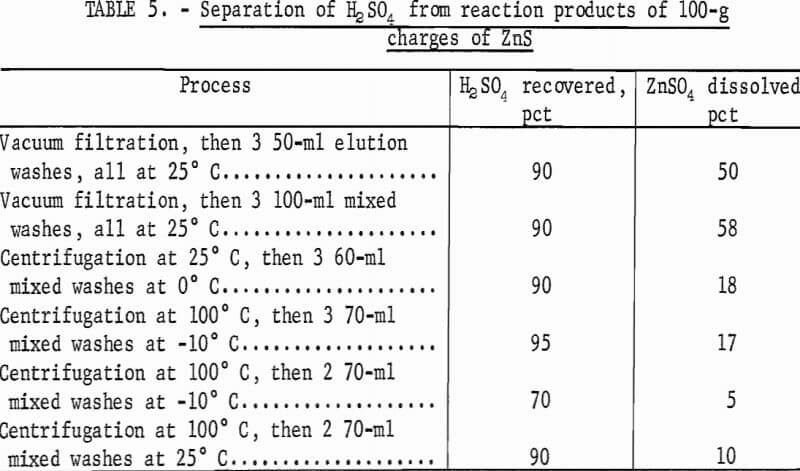
The flowsheet in figure 7 illustrates the laboratory method designed to minimize losses of zinc and dilution of the acid streams, and to produce neutral zinc sulfate for electrolysis. To recover moderately concentrated acid in the initial separation, the reacted ZnSO4-H2SO4 mixture was filtered or centrifuged, and then washed by mixed washes (mixed with the solids) or elution washes. Various combinations of these operations were tested; the results are given in table 5. The last process in table 5 was chosen for most of the laboratory work.
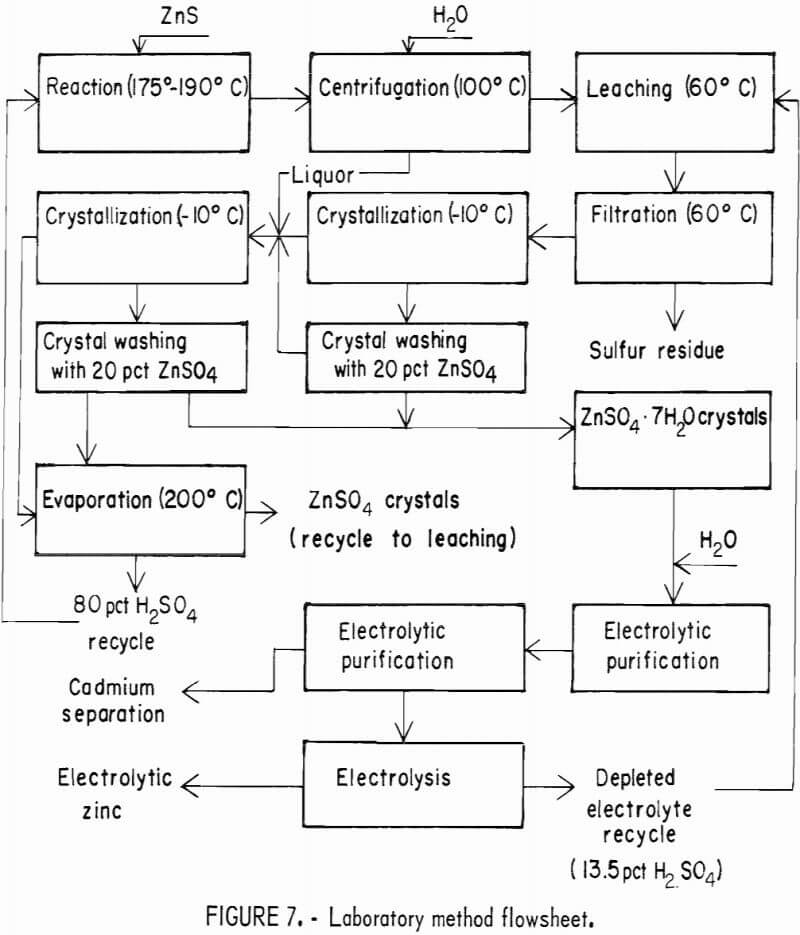
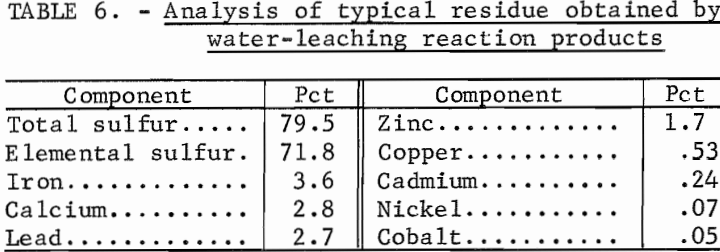
Simulated spent zinc electrolyte solution was used to leach the solids from the centrifugation step. Filtration was then used to separate the sulfur and unreacted solids from the ZnSO4-H2SO4 solution. An analysis of a typical leach residue is given in table 6. The filtrate was cooled to crystallize ZnSO4·7H2O. Yields were dependent on the final temperature of the solution, and as much as 80 pct of the ZnSO4 was crystallized at -10° C. The concentration of H2SO4 in the crystal liquor increased as ZnSO4·7H2O crystallized because of the water of hydration in the crystals. Leach solutions with an initial H2SO4 content of 22 pct contained 40-pct H2SO4 after crystallization of ZnSO4-7H2O; this concentration results in a significant reduction in the cost of recycling the acid back to the leaching step.
The solubility of ZnSO4 in H2SO4 solution decreases as temperature decreases and as acid concentration increases. The crystal form of ZnSO4 in equilibrium with ZnSO4 solution is ZnSO4·7H2O up to 38° C and ZnSO4·6H2O from 38° to 60° C. Above 60° C, zinc sulfate crystals are less hydrated. Increasing the H2SO4 concentration decreases the dehydration temperature and the solubility of ZnSO4. The triple point of equilibrium between ZnSO4·7H2O and ZnSO4·6H2O occurs at 18° C and 25 pct H2SO4; the solution contains 21.3 pct ZnSO4.
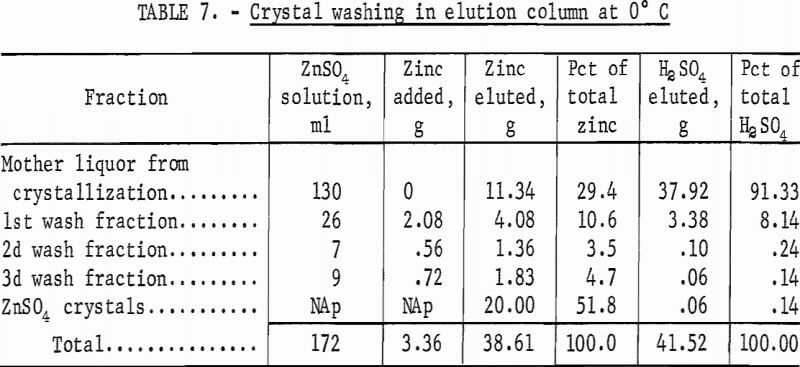
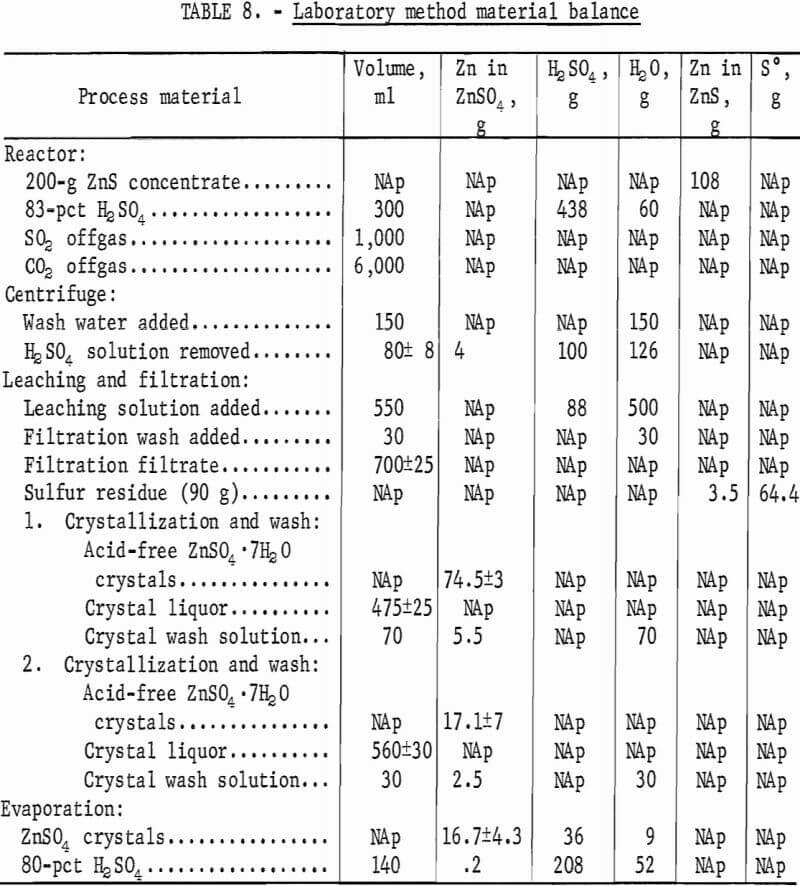
The product consisted of dense, granular crystals of ZnSO4·7H2O that readily settled from the mother liquor. However, a considerable amount of acid remained on the crystals after filtration. Elution washing in a column 2 cm ID by 50 cm long with a 20-pct ZnSO4 solution at 0° C removed the adhering acid. Table 7 gives the results of elution-washing tests; 99.5 pct of the acid was removed by washing with a quantity of neutral ZnSO4 solution equivalent to about 10 pct of that produced by the process. The ZnSO4 crystals were dissolved in water, and the solution was purified and electrolyzed. The acid filtrate from the crystallizers was concentrated by evaporation and recycled. A material balance obtained from a series of 12 tests is given in table 8.
Purification and Electrolysis of ZnSO4 Solution
The ZnSO4 from the crystallization step was dissolved in water to form a near-saturated solution of about 200 g/l for electrolysis. Iron was removed by precipitation as Fe(OH)3 (ferric hydroxide) from a neutral solution after oxidation with KMnO4 (potassium permanganate). The permanganate also caused the manganese in solution to precipitate as MnO2 (manganese dioxide). The iron precipitation is also effective in removing traces of antimony, which is detrimental to the electrolysis of zinc.
Up to 2 g/l of CuSO4 (copper sulfate) and 20 mg/l of As2O3 (arsenic tri-oxide) were added to the solution after it was heated to 90° C. High-purity zinc dust then was added at intervals for 1 hour to cement out Cu, Co, As, and Ni. Cobalt and nickel contents each were reduced from about 400 ppm to less than 1 ppm by this treatment. Excessive amounts of manganese in solution interfered with complete cementation of cobalt. In some cases, the iron precipitation and the addition of zinc dust had to be repeated to obtain solutions sufficiently pure for electrolysis. Excessive amounts of cobalt and nickel in solution cause deposits of zinc to redissolve after several hours of electrolysis.
The purified solutions were electrolyzed in test cells using aluminum cathodes and platinum anodes, with a current density of 5 to 10 A/dm². Dense, coherent zinc deposits were obtained in 24-hour tests. The current efficiencies were over 90 pct, and no dissolution of the deposits was observed.
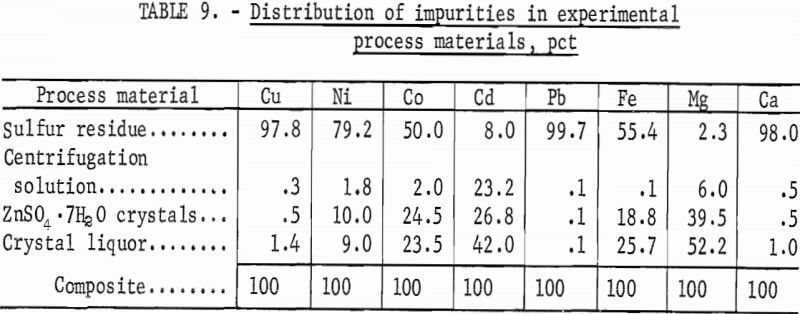
In continuous operation, minor impurities often build up to high levels. Intermediate products were analyzed to determine the distribution of impurities from the ZnS concentrate (table 9). Most of the Cu, Pb, and Ca, and about one-half of the Fe, remained in the sulfur residue. Cadmium, which is often a byproduct of zinc processing, is not lost and could be recovered by commonly employed purification processes. Iron, cobalt, and nickel also are removed by standard purification procedures. Magnesium would build up in process streams and would have to be removed by preleaching with dilute acid.
Summary
Sphalerite concentrates were reacted at ambient pressure with sulfuric acid to produce ZnSO4 and S. Sulfur was produced in the ratio of 4 moles for 3 moles of ZnS reacted under optimum conditions. Sulfur dioxide gas was produced when the acid concentration was excessive, and H2S was produced when the acid was dilute. When 80- to 85-pct H2SO4 was used, small, varying amounts of SO2 and H2S were produced during the reaction; the quantity varied with temperature. The reaction rate of finely ground ZnS concentrate is temperature dependent, and the reaction has an activation energy of 17.4 kcal/mole. More than 98 pct of the zinc was recovered as soluble ZnSO4 by reaction at 175° C for 3 hours.
The soluble reaction products were separated from sulfur and other insoluble residue by leaching with recycled electrolysis solutions containing 12 to 15 pet H2SO4. Zinc sulfate was separated from the H2SO4 by crystallization at -10° C as ZnSO4·7H2O, which removes water from the leach solution and concentrates the H2SO4 up to 40 pct. The H2SO4 then can be concentrated to 80 pct by evaporation and recycled to react with fresh ZnS concentrate. Neutral ZnSO4·7H2O was obtained by elution washing of the crystals with saturated ZnSO4 solution.

