LIX63 in combination with DNNS selectively extracts nickel and/or cobalt from acidic leach liquors with pH values as low as 1.0. Nickel/cobalt and cobalt/nickel selectivities are achieved by varying the relative concentrations of LIX63 and DNNS. Increasing LIX63 concentration retards both extraction and stripping rates while DNNS has the opposite effect. Slope analyses of equilibrium data indicate that both LIX63 and DNNS are present in the extracted complexes.
Experimental
LIX63 was used as received from General Mills. DNNS was prepared by repeatedly contacting the commercial product NaSUL-AS (trade name for the ammonium salt of dinonylnaphthalene sulfonic acid, marketed by R. T. Vanderbilt) with 600 gpl H2SO4 until titration showed that conversion to the acid form was complete. To mixtures of the two extractants, Solvesso 150, a product of Exxon, was added as diluent.
The nickel loading tests were performed with an aqueous solution (4.2 gpl Ni, pH 1.1) obtained by sulfuric acid leaching of laterite ore. Using conventional shakeouts, each organic mixture was loaded at O/A = 1/5 for 60 minutes. The long contact time and high A/O ratio were used to ensure that each mixture was loaded to capacity. Cobalt loading tests were performed with a synthetic cobalt sulfate solution (4.2 gpl Co, pH 1.1). Ten minute shakeouts were used at O/A = 1/5. Portions of the loaded organics resulting from the extraction tests were saved for the stripping experiments. Using 450 gpl H2SO4, the loaded organic mixtures were contacted at O/A = 1/1 for ten minutes. For the selectivity tests, laterite leach liquor (4.2 gpl Ni, pH 1.1) was spiked with Co and Fe to give 4.2 gpl each. Ten minute shakeouts were used at O/A = 1/5.
The rate studies used solvent mixes containing 30 v/o LIX63 and 5, 7.8, 13 and 15.5 v/o DNNS. Laterite leach liquor (4.3 gpl Ni, pH 2) was utilized for the extraction tests; stripping solution was 450 gpl H2SO4. Both the equilibrium and rate studies were performed at room temperature (O/A = 1/1).
Results
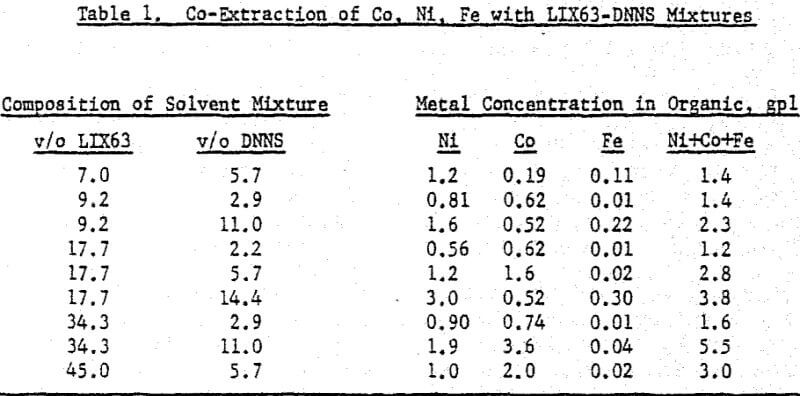
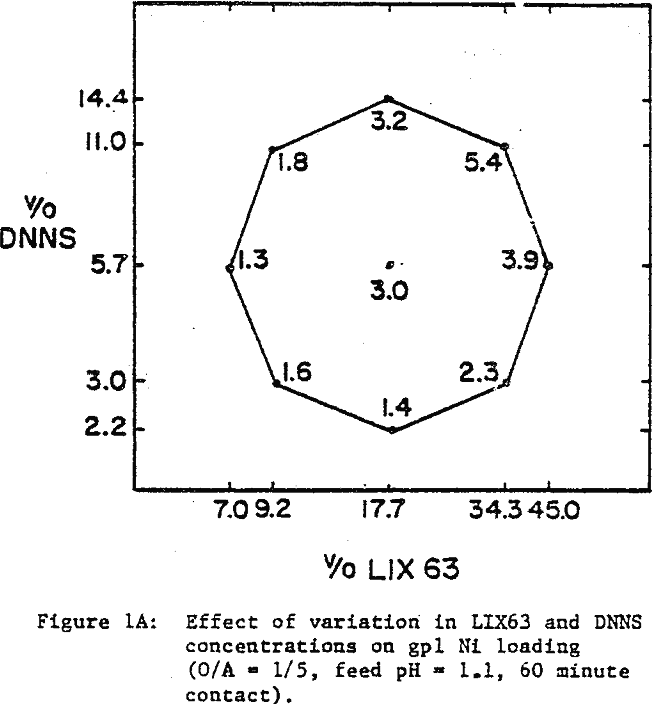
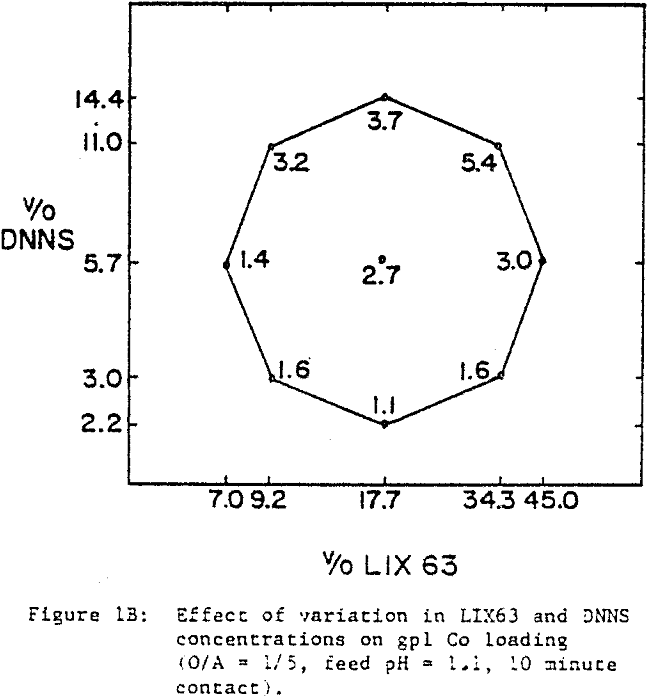
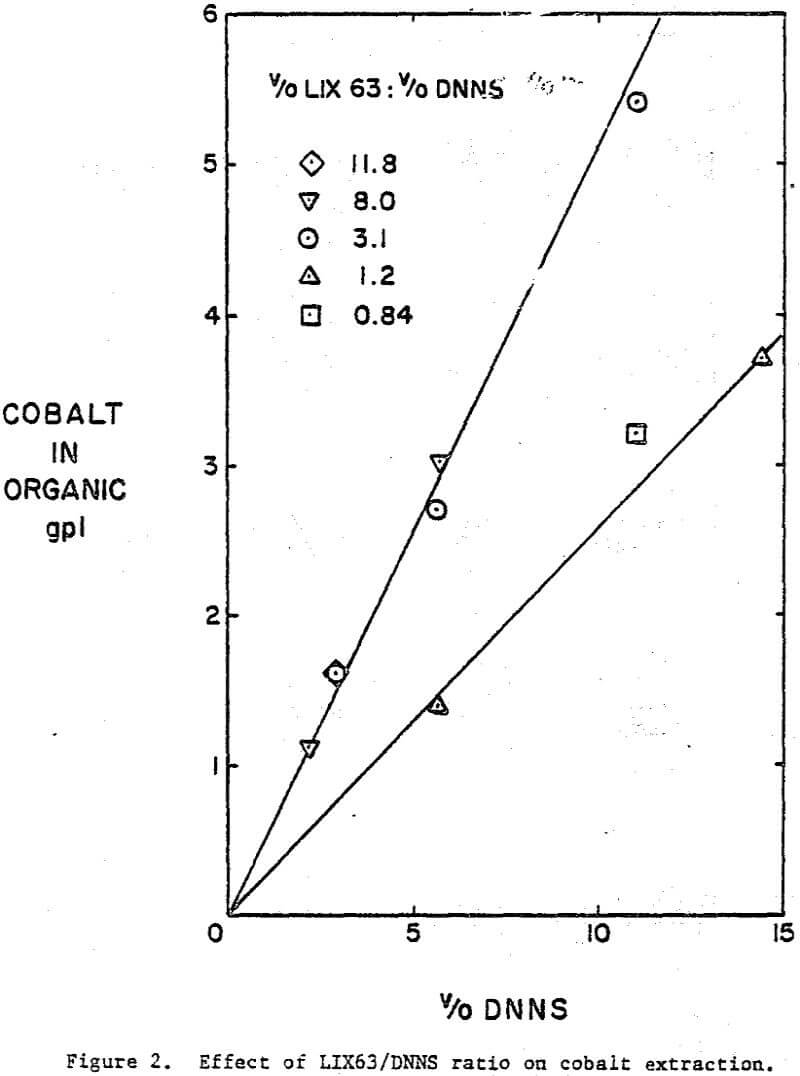
A comparison of the extraction data indicates that cobalt loading from cobalt-rich solutions is very similar to nickel loading from nickel-rich solutions. Loading is highest when LIX63 and DNNS concentrations are increased simultaneously. However, at a given DNNS concentration, loading increases with increase in LIX63 concentration until the ratio of v/o LIX63 to v/o DNNS reaches 3; beyond this ratio further increase in LIX63 concentration achieves no further improvement in loading.
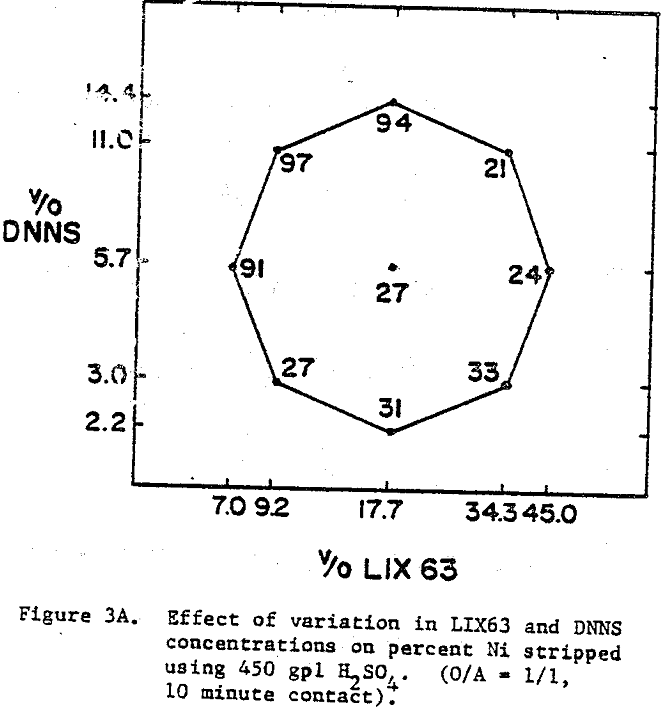
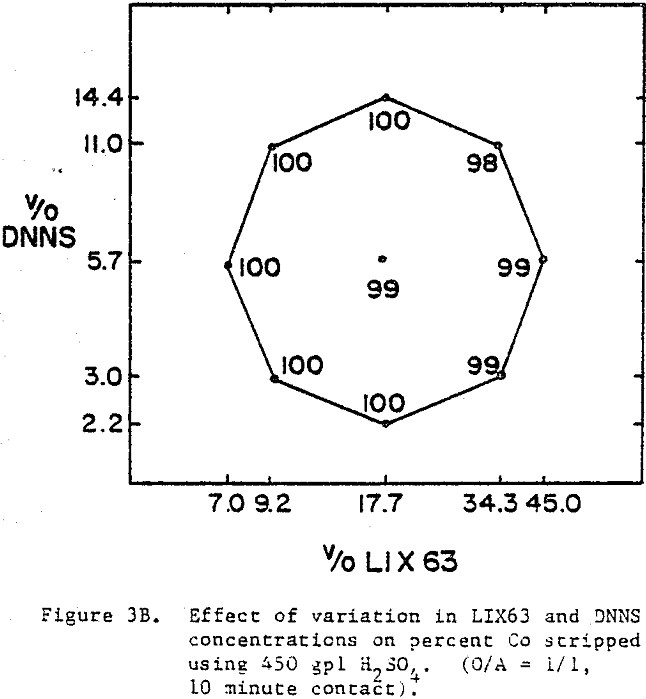
The best nickel stripping conditions (over 90%) are found in the narrow region located in the top left hand corner of the experimental region, i.e., high DNNS and low to intermediate LIX63. On the other hand, the cobalt stripping data indicate that cobalt strips readily (≥98%) from all the mixtures.
The “optimum” solvent mixture 16.3-14 v/o given by the RSM program, is based on prediction equations which are approximations of the true response surface. Therefore in order to discover the true optimum, various solvent mixtures in the neighborhood of the 16.3-14.4 v/o mixture were explored. Stripping Isotherms were determined for six extractant mixtures in which DNNS was kept constant at 15 v/o and LIX63 was varied from 30 v/o to 14 v/o.
Cobalt loading from cobalt-rich solutions is comparable with nickel loading from nickel-rich solutions. However when the two metals are present together, they behave differently. At a given DNNS concentration, the organic affinity for Co increases at the expense of Ni as the LIX63 concentration increases. On the other hand, it can be seen that as the DNNS concentration increases, nickel loading increases relative to that of cobalt.
The mixed LIX63-DNNS solvent extraction system can be used to process nickel-cobalt streams of widely varying nickel/cobalt ratios simply by altering the mixture composition. The following examples are presented to illustrate possible configurations for practical solvent extraction processes. These examples by no means exhaust the possibilities.
The extraction process can be visualized thus:
Ni++ + mHD + nHL ↔ NiDmLnHm+n-x + xH+
where HD and HL represent respectively, DNNS and LIX63, and NiDmLnHm+n-x is the extracted metal complex. Once we know x, m, and n, we can determine the form of the extracted complex
NiDmLnHm+n-x
For example, if n=o, it would mean that LIX63 is not involved in the complex. The standard method of determining x, m and n is the technique of slope analysis.