In the upgrading of finely-disseminated iron ores, selective desliming is the critical step which must be controlled in order to achieve efficient flotation. A prerequisite for selective desliming is a properly-dispersed pulp; sodium silicate is commonly used as a dispersant. The mechanism by which sodium silicate acts as a dispersant in the presence of calcium ions was examined by streaming potential measurements, settling tests, abstraction density determinations, selective flocculation tests and scanning electron microscope observations.
Experimental Results
Studies to delineate the manner in which sodium silicate affects selective flocculation of goethite-quartz mixture were carried out employing different experimental approaches. Initially, attempts were made to investigate the adsorption behaviors of Ca++ and silicate, but the unreliability of Ca and Si values obtained by atomic adsorption in the present system ruled out this approach.
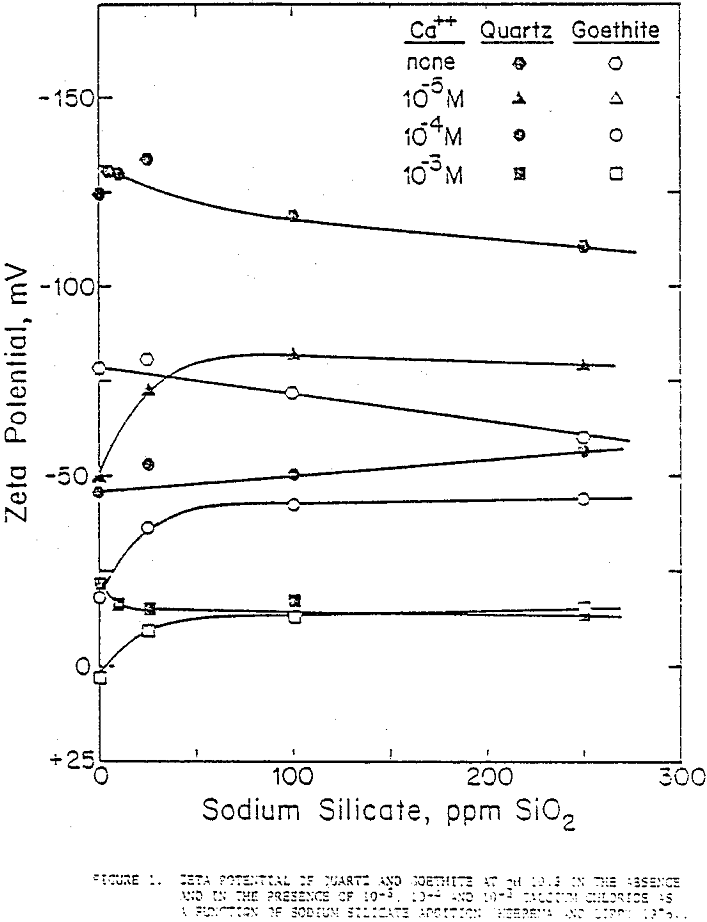
Streaming potential measurement results on quartz and goethite as a function of sodium silicate additions at 0, 10 -5M, 10 -4M, and 10-³M concentrations of CaCl2. In the absence of Ca++ the zeta potentials of quartz and goethite decreased gradually with sodium silicate concentration indicating that silicate ions acted, more or less, as indifferent ions towards these minerals.
With calcium ions in solution, there seemed to be some interaction as the zeta potential, lowered markedly by the adsorption of calcium ions on the quartz surface, moved up as these calcium ions attracted silicate anions but not sufficiently to bring the zeta potentials back to the levels of the calcium-free solutions.
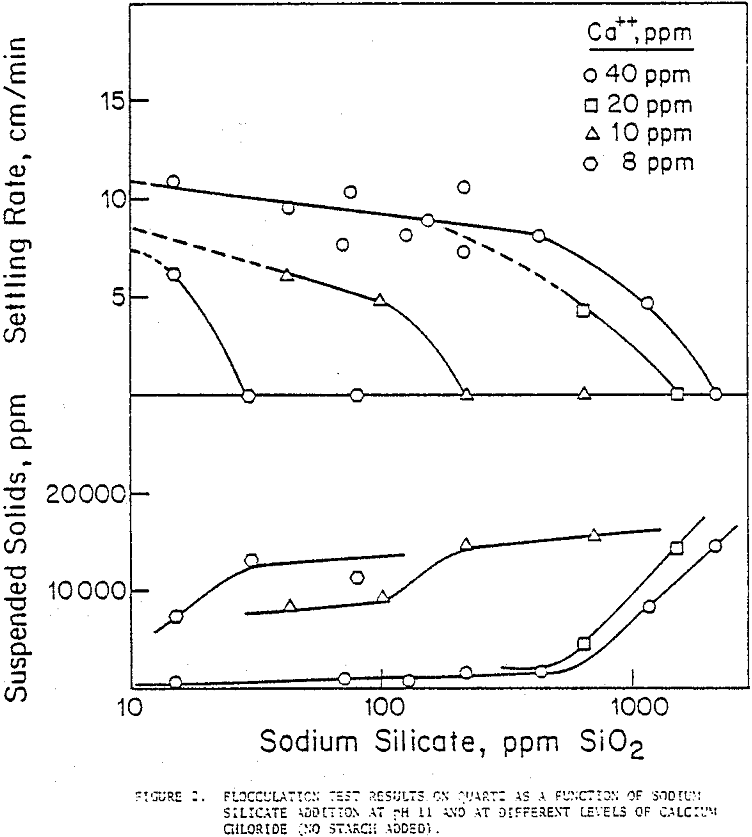
The effectiveness of sodium silicate in counteracting the adverse effects of Ca++ in dispersing the suspension was examined by a series of flocculation tests. Settling rates of quartz were observed to decrease as silicate additions increased at every concentration of Ca++ added. The lower the amount of Ca++ in the suspension, the lower was the silicate concentration that dispersed it fully; to illustrate, in the presence of 8 ppm Ca++, a sodium silicate addition of 30 ppm SiO2 dispersed the quartz suspension, whereas at 40 ppm Ca++ the corresponding addition of silicate needed was more than 2000 ppm SiO2.
To study the interactions of such a precipitate with quartz surfaces abstraction tests were carried out by changing the sequence of reagent addition in the following manner. In one case, calcium chloride was added to quartz followed by the addition of sodium silicate. In the other, calcium chloride and sodium silicate were mixed to form precipitates and then added to a suspension of quartz. These sequences are represented as Qz-Ca-Si and Ca-Si-Qz, respectively. The precipitates of calcium silicate and any adsorbed Ca++ on the surface of quartz were leached with hydrochloric acid and the leachate analyzed for the calcium abstracted.
Selective Flocculation Tests
A Ca++ addition of 10 ppm was chosen because flocculation tests, on quartz showed that considerably higher amounts of sodium silicate, involving higher ionic strength and the presence of colloidal silicate complexes, would be required to disperse quartz suspension containing more than 10 ppm Ca++. By increasing additions of sodium silicate adversely affected the selectivity. At a sodium silicate concentration of 400 ppm SiO2, the pulp was fully and indiscriminately dispersed. Tests performed at higher calcium concentrations and correspondingly higher sodium silicate additions also resulted in full dispersion of the pulp.
In the mining and mineral processing industry, common applications of sodium silicates are in the dispersion of siliceous gangue and slimes, clay mining and refining, the production of clay slips, ore flotation, stabilization of drilling muds, and surface conditioning of a variety of ores. At pH 11, for concentrations up to 600 ppm SiO2, the stable silicate species are monomeric. Above 600 ppm SiO2, multimeric ions along with in¬creasing amounts of “colloidal” silicates are present in addition to monomeric silicates.
By means of streaming potential measurements, they showed that the addition of starch to suspensions of goethite containing 10-³M CaCl2 and different concentrations of polyphosphates at pH 11, brought about only small changes in zeta potentials under those conditions where calcium polyphosphates were expected on the mineral surfaces. In the absence of any dispersants, the addition of starch caused an appreciable change in the zeta potential of goethite.