Table of Contents
- Experimental Procedure
- Experimental Results
- Effect of Iron Compound Type
- Effect of Iron-to-Molybdenum Ratio
- Effect of Ferric Sulfate Concentration
- Effect of pH
- Effect of Agitation Intensity
- Effect of Temperature
- Effect of Precipitator Retention Time
- Effect of a Retention Tank
- Effect of Two-Stage Precipitation
- Effect of Additional Flocculation
- Effect of Flotation Air
- Recycle Rate
- Air Pressure
- Froth Dewatering
- Deportment of Added Iron
- Solids Disposal
- Demonstration Runs
- Flow Diagram and Economic Evaluation
The minerals industry uses large volumes of water in many of its operations. Because of the rapidly decreasing availability of water in many areas and the need to control pollution, efficient and economical processes for purifying mineral waste waters are urgently needed. As part of a continuing program to devise technology for abating pollution caused by minerals processing, research was undertaken by the Bureau of Mines to remove traces of dissolved molybdenum from molybdenum concentrator waste water.
The molybdenum problem was reviewed by Ramadorai and Gott Molybdenum in soils and irrigation water is concentrated by some plants and may become a hazard to grazing animals. Levels of 1 or 2 ppb are not uncommon in rivers; Colorado River water is reported to contain 7 ppb of molybdenum. A rather high concentration of 25 ppm was found in one stream in the State of Colorado.
Molybdenum is important as a trace nutrient in the life processes of plants and animals, but its function is unknown. Acute or chronic dosages of molybdenum produce a toxicity comparable to that of potassium. Molybdenum, unlike lead and arsenic, is not cumulative but is rapidly excreted.
A mine and mill of the Molycorp, Inc., are located at Questa, N. Mex. The molybdenum mill discharges nearly 3,500 gpm of waste water plus tailings to a pond about 8.5 miles from the mill. Decanted water drains into the Red River, flowing through a short, deep canyon into the Rio Grande River. The pH 7.5 decant water contains, typically, in parts per million, 1,000 SO4, 320 Ca, 40 Na, 35 Mg, 10 Cl, 2-4 Mo, 2 F, 0.9 Mn, 0.16 Fe, and 0.06 Zn. If the molybdenum in the waste water is decreased to less than 0.6 ppm, the mill will not exceed the local 1979 waste water quality standards, which limit the daily discharge to 25 pounds of molybdenum. However, this level is not easy to achieve because conventional lime treatments, adequate for removing many heavy metals, fail to remove traces of molybdenum. The lack of proven practical technology for reducing molybdenum in waste water to acceptable levels prompted laboratory work at the Bureau’s Salt Lake City Research Center to devise and appraise an effective technique.
Recycling the waste water has also been considered; however, the effect of recycled water on concentrate grade and recovery is unknown. In addition, the cost for pumping water from the pond back to the mill would be appreciable. Nevertheless, the possibility of recycling was explored in the test program.
Several molybdenum removal methods were tested briefly to develop a feel for applicability to the problem. Neither sulfide, precipitation nor solvent extraction with amines was particularly effective at low molybdenum concentrations, and either method introduced objectionable impurities. Sorption using either activated carbon, ion exchange resins, or hydrated titanium oxide, although effective, appeared expensive. Coprecipitation with ferric hydroxide, a method well known in the field of analytical chemistry, was chosen because the method had been tested for use in uranium refining and appeared promising for treating waste water. LeGendre and Runnells worked on this method to explain the mechanisms involved in removing iron from natural waters. They pointed out that the mechanics enabling molybdenum removal by precipitation of ferric hydroxide are unknown, but three mechanisms are possible: (1) mechanical coprecipitation of molybdenum ions with ferric hydroxide, (2) adsorption of bimolybdate ions by ferric hydroxide, or (3) precipitation of an iron-molybdenum compound. Although the iron precipitation method proved effective during Bureau testing, determining the mechanism was outside the scope of the research program.
Experimental Procedure
A continuous laboratory test unit was used for removing molybdenum from water, using freshly precipitated ferric hydroxide. The continuous unit (figs. 1-2) was used to simulate conditions that might occur in an industrial application and that would provide sufficient froth for dewatering tests. The equipment was comprised of two stages. Chemical additions resulting in iron precipitation and subsequent flocculation preceded dissolved-air flotation.
In the precipitation stage, both water and 0.05M ferric sulfate were metered to the precipitation vessel, a 2.5-liter beaker. Baffles and a turbine mixer provided good mixing. Precipitation was controlled by adding either a 5 pct lime suspension or 1N H2SO4 managed by a pH controller. Precipitator effluent flowed into a 3.5-liter retention vessel where flocculation occurred.
Additional flocculation was achieved in a 0.6-liter beaker where flocculant was dispersed with an agitator. After this procedure, the suspension was treated in the flotation unit.
The technique of dissolved-air flotation is not well known in the metallurgical industries, although the method has been used for many years to remove low-density materials from waste waters. Dissolved-air flotation uses very fine bubbles for ore flotation. The bubbles, appearing as a fog, are formed when pressurized water saturated with air is released to atmospheric pressure. The experimental equipment used a recycle flow system in which clarified effluent was pressurized and saturated with air, the aerated water was then mixed with unclarified water. This system is used when pressurizing unclarified water would destruct the flocs.
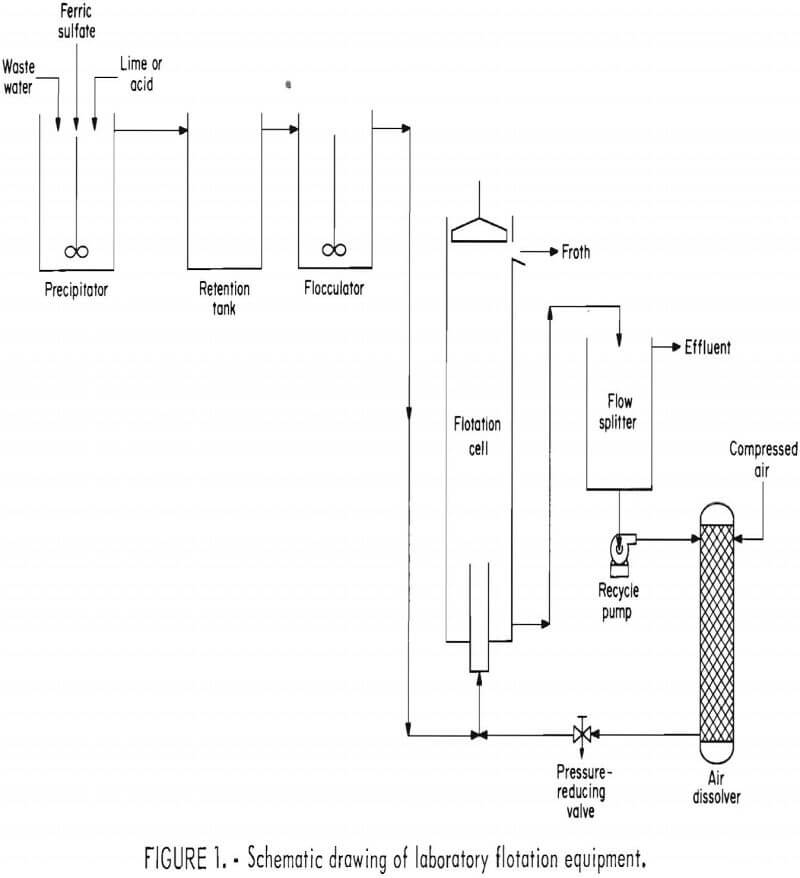

The experimental flotation unit was a 5-foot-high, 6-inch-diameter glass column with a 2-inch-diameter by 1-foot-long mixing chamber in the bottom. Clarified effluent was withdrawn from the bottom of the column beneath the influent inlet. Part of the clarified effluent was pressurized and saturated with air in a packed column at 50 psig. The pressurized effluent was vented through a needle valve and mixed with treated waste water in the flotation column mixing chamber. Very fine bubbles (fig. 3) attached to the precipitate and collected on the top as a froth. The froth was discharged from the top of the flotation column by means of a rotating scraper.
Operation, of the flotation unit at 1 liter/min resulted in retention of the suspension for 2.5 min, 3.5 min, and 0.6 min in the precipitator, retention tank, and flocculator, respectively. Precipitation conditions were, unless otherwise noted, ferric sulfate addition at 0.05M, a pH of 5, and temperature of 15° C. Normally, 30 pct of the clarified effluent was pressurized and recirculated to the flotation unit. Agitation power input to both the precipitator and the flocculator was mild and equivalent to 0.1 hp/1,000 gal. The various operating conditions are discussed in detail in following sections of this report.
Traces of molybdenum in the effluent were determined directly by means of an inductively coupled argon plasma spectrometer. Determination was made using the molybdenum line at a wavelength of 2,038.44 A.
Experimental Results
A variety of operating conditions were examined to maximize molybdenum removal at minimum cost. The effects of the operating variables were determined by assaying samples of flotation cell underflow taken after 4 hours of steady operation. Samples were taken hourly for 3 hours, and the results were averaged. Soluble molybdenum was determined by analysis of samples filtered through a 0.2-µm Millipore filter. Total molybdenum was determined after acidifying the unfiltered sample to dissolve particulate matter.
Effect of Iron Compound Type
The degree of molybdenum removal was unaffected by whether ferric iron was added as a solution of the chloride or of the sulfate. Ferric sulfate was preferred because it was less expensive. Ferric, rather than ferrous, iron was used because ferric iron is a more effective scavenger for molybdenum, and ferric iron is least soluble at the operating conditions employed.
Effect of Iron-to-Molybdenum Ratio
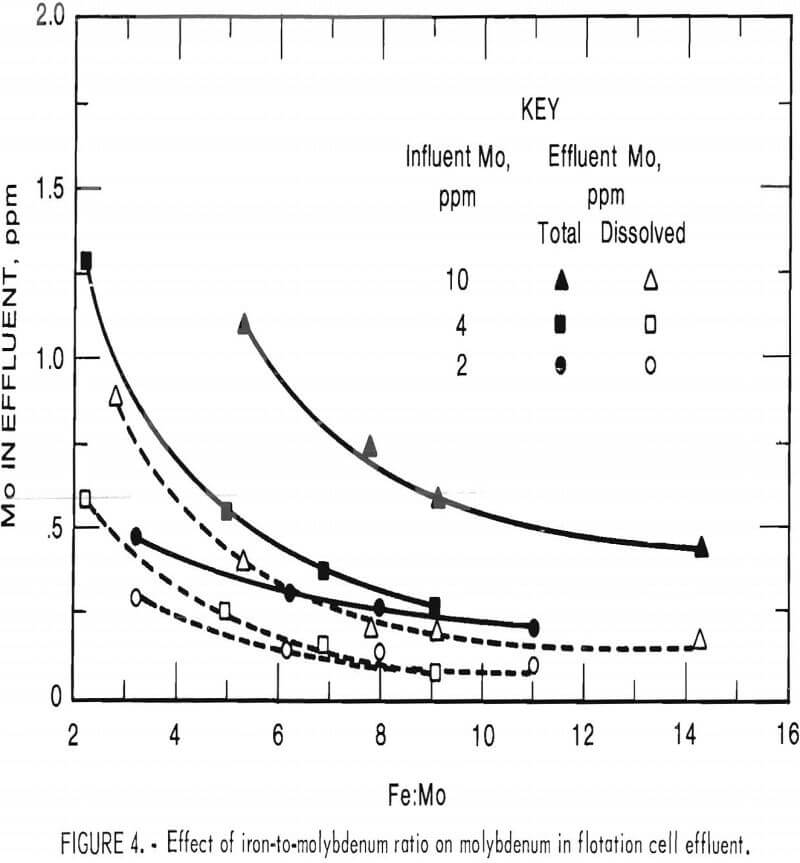
The amount of ferric iron addition, expressed as a ratio of iron to molybdenum, determined the degree of molybdenum precipitation from simulated waste water spiked with hexavalent molybdenum to levels of 2, 4, and 10 ppm. Both total and soluble molybdenum concentrations in flotation cell underflow are shown in figure 4. Reduction of the total molybdenum to 0.5 ppm required iron-to-molybdenum ratios of 3.2, 5.4, and 12 for water initially containing 2, 4, and 10 ppm Mo, respectively. Unless noted otherwise, other tests were carried out using these iron-to-molybdenum ratios.
Effect of Ferric Sulfate Concentration
The effect of the precipitant concentration on molybdenum removal was compared at ferric sulfate concentrations of 0.05M and 0.5M, with simulated waste water containing 10 ppm Mo, and an iron-to-molybdenum ratio of 12. Best results were achieved using the more dilute ferric solution. Total molybdenum in the flotation effluents with 0.5M and 0.05M ferric sulfate averaged 0.86 and 0.5 ppm, respectively. Unless otherwise noted, other conditions were tested using 0.05M ferric sulfate.
Effect of pH
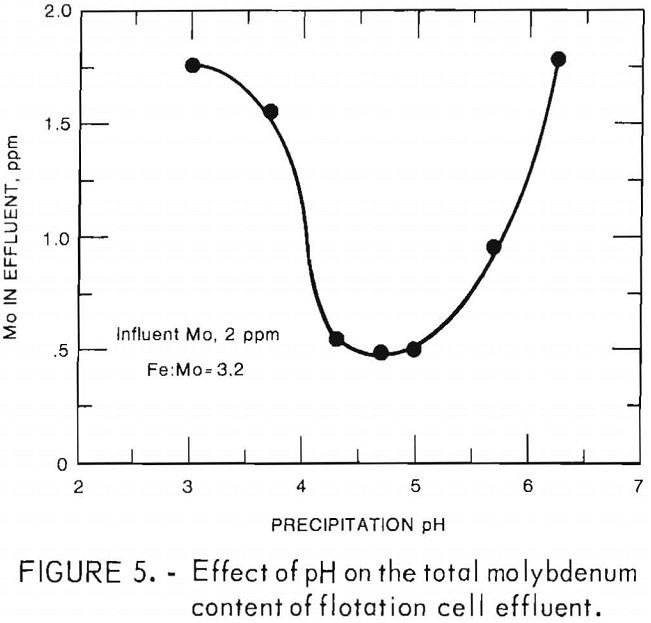
Maximum molybdenum removal was achieved by operating between pH 4.2 and pH 5.0. The test results shown in figure 5 were achieved at an initial molybdenum concentration of 2 ppm and an iron-to-molybdenum ratio of 3.2. The trend of these results was confirmed at molybdenum concentrations of 4 and 10 ppm. Testing of other variables was done at pH 5 unless otherwise noted.
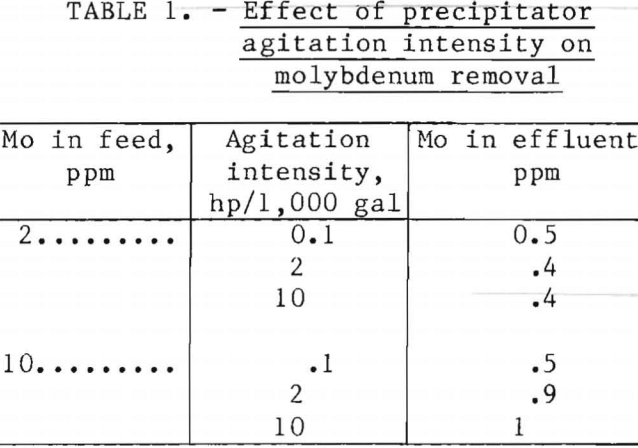
Effect of Agitation Intensity
The effect of precipitator agitation intensity on molybdenum removal was tested using water containing either 2 or 10 ppm Mo. Iron-to-molybdenum ratios of 3.2 and 12 were employed at the low and high molybdenum concentrations,
respectively. Power inputs were determined using the method described by Rushton and Oldshue. Total molybdenum remaining in flotation cell effluent is shown in table 1. Intense agitation was detrimental to molybdenum removal from water initially containing 10 ppm Mo, whereas this trend was not apparent using water initially containing 2 ppm Mo. Molybdenum precipitated from water initially containing 10 ppm Mo was nearly 98.5 pct, regardless of agitation intensity. The percentage of the molybdenum floated from 10-ppm-Mo feed decreased from 95 to 92 when the agitation intensity increased from 0.1 to 10 hp/ 1,000 gal.
Effect of Temperature
Molybdenum removal from water initially containing 2 ppm Mo was not influenced by temperatures ranging from 10° to 25° C. Molybdenum removal was significantly reduced, however, at 50° C, an elevated temperature not expected to be encountered in mill effluent. For example, 76 pct of the molybdenum was removed at 20° C, whereas only 51 pct was removed at 50° C.
Effect of Precipitator Retention Time
The length of time that the feed was retained in the precipitator, ranging from 48 sec to 2.5 min, had no noticeable effect on molybdenum removal when the feed initially contained 10 ppm Mo. Retention time was varied by changing the precipitator size. Agitation intensity was held constant at 0.1 hp/1,000 gal.
Effect of a Retention Tank
Preliminary batch flotation tests showed that the most effective flotation was achieved when the suspension contained large flocs. A finely divided precipitate was formed in the precipitator, and flocculation occurred soon after the agitator was stopped. Adding a nonstirred retention tank after the precipitator allowed flocculation to proceed during continuous operation, and flotation was enhanced. For example, continous operation with feed initially containing 2 ppm Mo resulted in removal of 72 pct of the molybdenum when no retention tank was used, and 80 pct when 3.5 min of quiescent retention were used.
Effect of Two-Stage Precipitation
Precipitation in two stages in series, with ferric sulfate added in each stage, was not advantageous. Using waste water spiked with 10 ppm Mo, at an iron-to-molybdenum ratio of 7.5, total molybdenum in effluent from the flotation cell was 0.8 ppm with either one or two-stage precipitation.
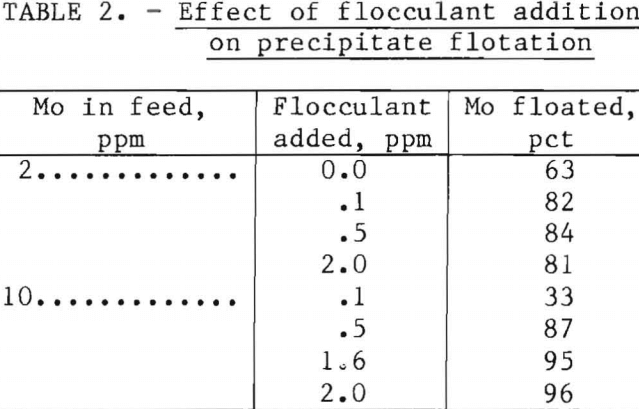
Effect of Additional Flocculation
Although the iron-molybdenum precipitate flocculated in the retention tank, additional flocculation using a nonionic polyelectrolyte, Superfloc 127, improved subsequent flotation. Preliminary batch tests with several anionic and cationic flocculants showed no particular advantage in the use of flocculants other than nonionic. Results of continuous flotation tests (table 2) show the improvements in molybdenum removal achieved by adding up to 2 ppm of flocculant. Maximum flotation using water initially containing 2 ppm Mo was achieved with 0.5 ppm of flocculant. Little, if any, advantage was achieved by adding more than 1.6 ppm of flocculant to water initially containing 10 ppm Mo.
Effect of Flotation Air
The quantity of air released in the flotation cell is an important design variable. A low air-to-solids ratio can cause poor flotation, and an overly high air-to-solids ratio will reduce the unit’s capacity and may also be deleterious to flotation. In laboratory tests, clarified effluent was saturated with pressurized air and recycled to the cell to provide flotation air. The amount of air released was proportional to the recycle rate, the water temperature, and the pressure. Air solubility in water is proportional to the pressure and inversely proportional to the temperature and, at normal operating conditions of 15° C and 50 psig, the air solubility was equivalent to 1 pound of air per 1,000 gal of water. Nearly 90-pct saturation was achieved in the air dissolver.
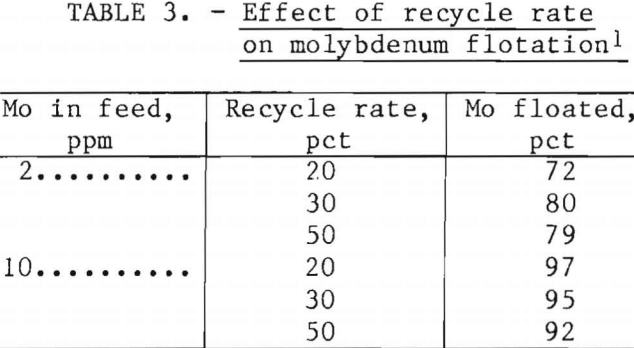
Recycle Rate
Flotation air was varied in a series of tests by using recycle rates of 20, 30, and 50 pct. Table 3 shows the ineffectiveness of a 20-pct recycle when water containing 2 ppm Mo was treated. Best flotation was achieved by recycling and pressurizing 30 pct of the effluent. When water containing 10 ppm Mo was treated, a recycle rate of only 20 pct was best. The greater efficiency of a relatively low air-to-solids ratio for water containing 10 ppm Mo appeared to be caused by the larger floccules resulting from increased iron addition.
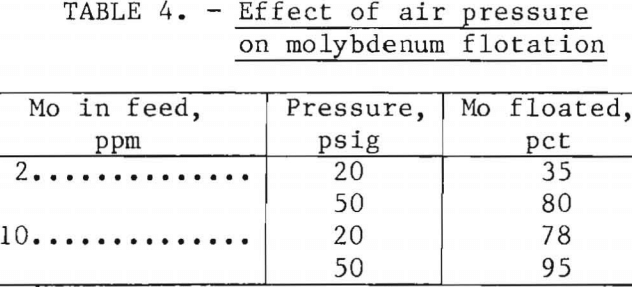
Air Pressure
Air was dissolved in recycled water at pressures of either 20 or 50 psig. Better molybdenum flotation, shown in table 4, was achieved at a pressure of 50 psig, a pressure frequently used in large-scale operations.
Effect of Flotation Cell Throughput
Total flow rates of feed plus pressurized recycle water, in commercial dissolved-air flotation units, generally range from 1 to 2 gpm/ft² of cross-sectional area. Waterflow through the laboratory unit routinely was 1.75 gpm/ft². Flotation tests at throughputs of 1 to 3 gpm/ft² showed no particular advantage in operating at either the highest or lowest rates. Test results are shown in table 5.
Froth Dewatering
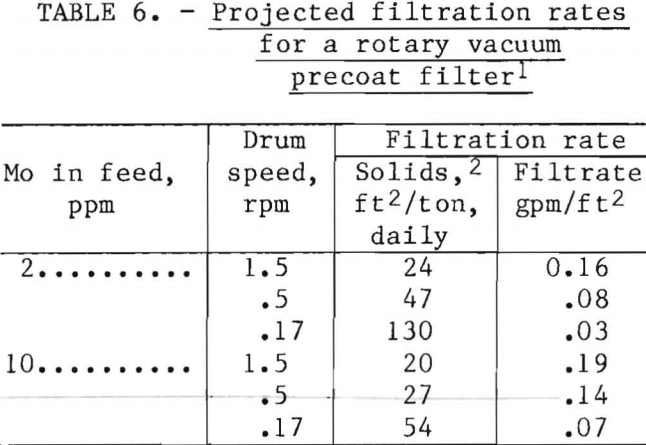
Dewatering the flotation froth to enable disposal of the precipitate as low-volume, moist solids proved troublesome. The finely divided precipitate required filters of small pore size to achieve a clear filtrate. The filter surface was rapidly blinded by compressible solids, and neither pressure filtration nor ordinary vacuum filtration appeared practical. Rotary vacuum pre-coat filtration frequently is employed for dewatering precipitates with troublesome characteristics, and this technique appeared workable in laboratory testing, using a vacuum test leaf to simulate filter operations.
Filtration results shown in table 6 were obtained using a 0.1-ft² filter leaf coated to a depth of 4 inches with diatomaceous earth. Filtration simulated a drum submergence of 37.5 pet at a vacuum of 15 in. Hg. A filter cake was produced containing 80 pct moisture with solids comprised of diatomaceous earth and iron-molybdenum precipitate. Filtration rate was proportional to filter rotation speed at the conditions tested.
Deportment of Added Iron
The low solubility of iron in nearly neutral, aerated water limits the soluble iron content in the treated waste water to 0.1 ppm or less. However, the presence of particulate iron in the effluent from the laboratory flotation cell resulted in total iron concentrations ranging up to 7 ppm. Legally permissible total iron concentrations in waste water are generally limited to 1 ppm or less, and, unless Molycorp can arrange with environmental control authorities for a variance to allow a higher concentration of insoluble iron, further clarification by filtration appears necessary as a final water purification treatment.
Solids Disposal
No decision was reached concerning the best means for disposing of the moist filter cake that, at a waste water concentration of 2 ppm, would contain nearly 60 pounds daily of molybdenum. The molybdenum concentration in the dry solids, estimated to be nearly 6 pct, was not accurately determined because of uncertainty about the degree of dilution with filter precoat. The filter cake could either be stabilized and buried with landfill or stored pending treatment to recover the molybdenum. Although preliminary testing appeared promising for molybdenum recovery, a recovery method was not developed in this phase of the research.
Demonstration Runs
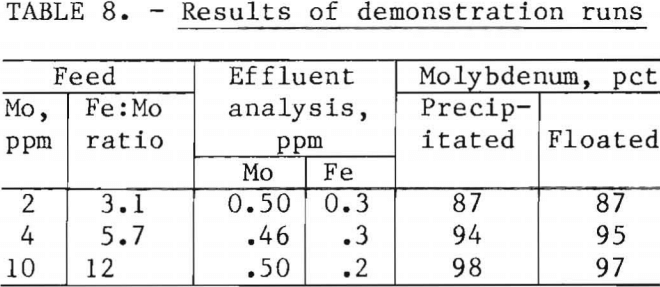
Demonstration runs were made using simulated waste water containing 2, 4,
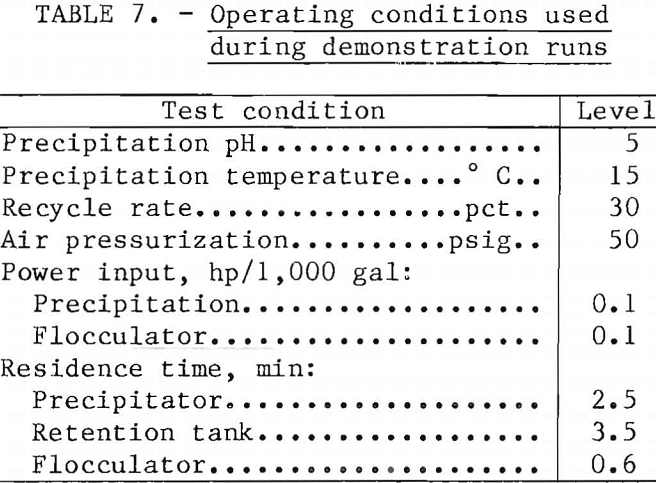
and 10 ppm Mo and the best molybdenum removal conditions determined during the testing program. The operating conditions are shown in table 7, and results are shown in table 8. Total molybdenum in the treated waters was 0.5 ppm or less, whereas total iron ranged from 1.7 to 7.3 ppm.
Flow Diagram and Economic Evaluation
A flow diagram showing equipment items and material flows for a conceptualized water treatment plant, projected from laboratory results, is shown in figure A-1 in the appendix. The process was designed to treat each of three waste streams discharged from the tailings pond: streams containing either 2 or 4 ppm Mo at 3,500 gpm, or a stream containing 10 ppm Mo at 700 gpm. This third option was achieved by recycling 2,800 gpm of pond overflow back to the concentrator. In this case, 2,800 gpm of water would be conserved and only 700 gpm of treated effluent discharged; however, pumping costs would be considerable. The effect of recycled water on concentrator performance is unknown.
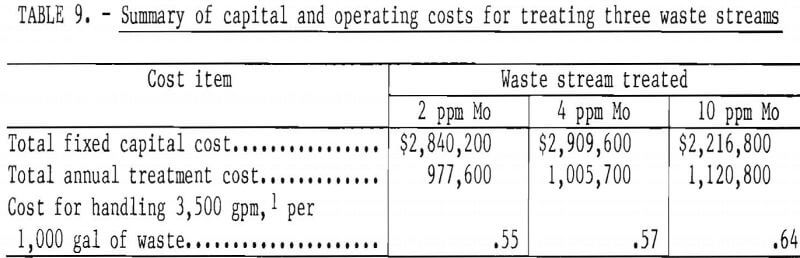
Summaries of capital costs and operating costs are in table 9. Estimated costs for treating 3,500 gpm of water containing either 2 or 4 ppm Mo were 55 cents and 57 cents per 1,000 gal, respectively. Thus, doubling the molybdenum concentration at this level had little effect on water treatment costs. At 10 ppm Mo, water treatment costs increased to $3.18 per 1,000 gal; however, only 700 gpm of water were treated. Recycling 2,800 gpm of untreated water was estimated to cost 30 cents per 1,000 gal, but this high pumping cost was tempered by a credit of 7.88 cents per 1,000 gal, the cost of fresh process water. Thus, the cost for treating and recycling a total flow of 3,500 gpm of water containing 10 ppm Mo was 64 cents per 1,000 gal, and this was the most expensive of the three options considered.
Conclusions
Molybdenum dissolved in simulated mill waste water was decreased from levels of 2, 4, and 10 ppm to less than 0.5 ppm by a method, demonstrated in the laboratory, that was comprised of coprecipitation with ferric hydroxide, followed by precipitate recovery by means of dissolved-air flotation, and rotary pre coat filtration. This water quality would meet proposed standards for molybdenum discharge of less than 25 pounds per day; however, the iron content could prove excessive. The iron content, as high as 7 ppm of ferric hydroxide precipitate, may be reduced to an acceptable level by sand filtration in the event that reduction is required.
Estimated cost for decreasing molybdenum from 2 ppm to 0.5 ppm was 55 cents per 1,000 gal of treated water.
Treatment costs for water containing 4 ppm Mo was 57 cents per 1,000 gal. Treating 20 pct of the waste water and recycling the remainder could raise the molybdenum concentration in the waste water to about 10 ppm. This operation was estimated to cost 64 cents per 1,000 gal of concentrator effluent. High pumping expense to return water from the distant tailings pond to the mill makes this idea unattractive. Items not included in the treatment costs are either an expense or a credit for disposing of the molybdenum-iron sludge plus a possible added expense for filtering the treated waste water to remove suspended iron. Credit for recovering the molybdenum would be less than the market value of the metal content or nearly 13 cents per 1,000 gal of water containing 2 ppm Mo.
