Table of Contents
As part of the mission of the U.S. Department of the Interior’s Bureau of Mines to help insure the continued viability of the domestic minerals and materials economy, and to help maintain an adequate minerals base, research was conducted to investigate in situ leach techniques for extraction of copper from a chalcopyrite ore. Successful application of in situ leaching to copper sulfide ores would enable exploitation of large copper resources that cannot be treated by conventional methods.
In situ leaching has a number of potential advantages over conventional mining and milling procedures. The advantages include—
- Lower energy requirements.
- Lower capital investment.
- Minimal permanent land disturbance.
- Less long-term pollution potential.
Because the ore remains in place and is not crushed or ground, the energy requirements are small. Elimination of crushing, grinding, and beneficiation equipment decreases the capital investment of the project. The landscape remains unspoiled because there are no open pits, waste dumps, or tailing ponds associated with in situ leaching. Absence of these structures eliminates the long-term potential for air pollution due to wind erosion and water pollution due to precipitation seeping through the mined area.
In situ leaching also has potential disadvantages when compared with conventional mining-milling practice. The disadvantages include —
- Lower metal recovery.
- Lower accessory element recovery.
- Long extraction times.
- Higher risk of failure.
- Waste leach solution disposal problems.
Nevertheless, when the advantages outweigh the disadvantages, in situ leaching has been and is being used.
In situ leaching is being practiced commercially in the uranium and copper industries. Uranium operations use injection and recovery wells drilled into the ore body. The well casings are perforated where they intersect the ore horizons so that the weak acid or base leach solutions flow only into ore. The sandstone host rock is porous to the leach solutions and requires no fracturing prior to leaching. The lack of fracturing is, in fact, often necessary for efficient uranium recovery. Leach solution is injected, flows through the ore formation, and is pumped out of the recovery wells. Uranium is extracted from the leach solution by ion exchange. The barren solution is adjusted to leach solution strength with makeup chemicals and reinjected in the ore body for further leaching of the uranium.
Copper has been leached in place at the Miami operation of Cities Service Co. since 1941. Dilute sulfuric acid solution is injected into the overburden and trickles through the block-caved mixed oxide-chalcocite-bearing rock. The solution collects in a sump at the base of the treatment zone and is pumped to a solvent extraction-electrowinning plant for copper recovery. The barren solution, adjusted to the required acid strength, is reinjected into the ore for further leaching.
Copper has been recovered commercially from oxidized deposits that were fractured in place by chemical explosives. The Ranchers Exploration Co. and McAlester Fuel Co. operations in Arizona are recent examples of the technology. Ranchers fragmented the Old Reliable hillside deposit with explosives, terraced the fragmented ore, and then leached it with dilute sulfuric acid. The solution percolated through the ore by gravity. Copper-bearing pregnant solution emerged at the bottom of the fragmented ore. Copper was removed from solution by cementation on iron. The Zonia deposit of McAlester Fuel was fragmented, and recovery wells were drilled to the bottom of the fragmented ore zone and cased. Acid solution was sprayed on the rubble, percolated down through the ore by gravity, and was recovered by pumping from the recovery wells. Copper was extracted from the solution by cementation. Occidental Minerals actively assessed the prospects of in situ leaching of the large oxidized Van Dyke copper deposit located beneath the town of Miami, Ariz. Preliminary testing of a five-spot well system after hydrofracturing was very encouraging. The company tested their technique with a nine-spot well pattern to assess the feasibility of scaled-up operations. The deposit probably cannot be exploited by means other than in situ leaching because of its location.
Low copper extractions have precluded commercial application of in situ leaching of unoxidized copper sulfide ores. Chalcopyrite ores are particularly resistant to leaching in situ. Previous research has shown that oxidizing acid solutions, such as dilute nitric, nitric-sulfuric, oxygen- or air-sulfuric oxygen- or air-hydrochloric, chlorine- hydrochloric, or ferric chloride or sulfate, and basic solutions of air- or oxygen-ammonia are capable of oxidizing chalcopyrite. However, most research on in situ leaching of chalcopyrite ore has been conducted with the oxygen-sulfuric acid system because some sulfuric acid is produced during oxidation of sulfide minerals in the ore. This acid is utilized in the leaching process, thus decreasing the amount of acid required to leach the ore. Ammonia-oxygen systems have also been investigated when basic gangue constituents are present in the ore.
Leaching of chalcopyrite ore has been scaled up to tests involving over 6 tons of minus 15.2-cm (6-inch) ore. These experiments were conducted for at least 700 days with sulfate solutions at 90° C. Oxygen at 2.8 MPa (400 psig) was also bubbled through the solution. No acid was added to the solutions because oxidation of the sulfides was sufficient to maintain the pH of the solution at 1.95. The copper extraction rate was parabolic during the first stage of the experiment and linear thereafter. Overall copper extraction was more than 45 pct. Research showed that the water- oxygen system for in situ leaching was technically feasible but required long leach cycles to achieve good extractions.
Kennecott Copper Corp. extended research on chalcopyrite ore to a field test. The company drilled a five-spot well pattern to a depth of 5,000 feet in a deeply buried portion of their Safford, Ariz., deposit. Although the results of the test are proprietary, the experiment was significant because of the great depth at which it was conducted.
Laboratory research was conducted to study the effect of alkali metal chloride leach solution additives on copper extraction from chalcopyrite ore crushed to less than 1.27 cm (0.50 inch). These studies showed a beneficial effect on the copper extraction rate at elevated temperatures owing to the formation of ferric chloride and the complexing ability of the chloride ion. However, there is doubt concerning the applicability of these results to larger sized ore because of the long diffusion paths for reactants and products into and out of the rocks when compared with those that existed in the previous effect-of-chloride-ion investigations.
This report is an extension of previous large laboratory in situ leach experiments on chalcopyrite ore with the purpose of determining if NaCl solution additives enhance the copper leaching rate at 90° C and 2.8 MPa (400 psig) of oxygen pressure when long diffusion paths are involved. A secondary purpose is to define the leaching reactions and the oxygen and sulfuric acid requirements for a leach system with and without the NaCl additive by calculating mass balances for each leach system.
Materials and Equipment
The ore used in the experiments was obtained from an Arizona porphyry copper deposit and contained

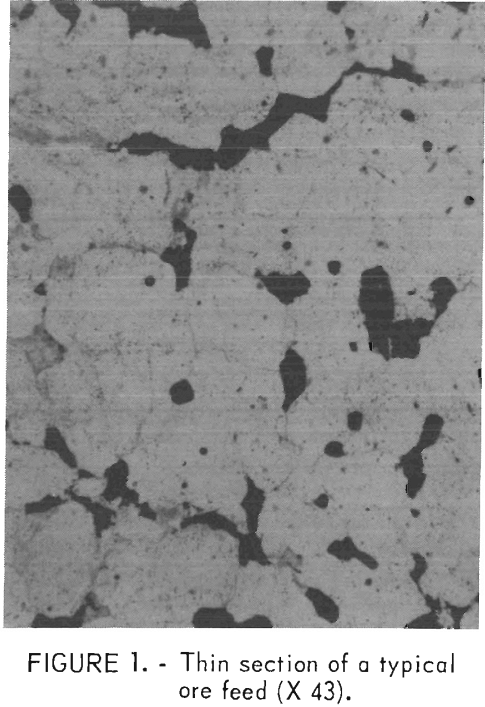
There were no discernible trends in the analyses as a function of particle size. However, there was unavoidable deviation from the copper head analysis in the larger size fractions owing to the small number of fragments comprising this portion of the feed. The copper mineralization was primarily chalcopyrite and some bornite. The major gangue mineral was sericite, and iron oxides were also present. The original porphyritic texture was preserved in some of the rock. The copper minerals were disseminated along the quartz grain boundaries. Pyrite was found mainly in veins, but some was also disseminated throughout the rock. Figure 1 is a photomicrograph of a thin section of a typical rock. The matrix grains are quartz, the black grains are chalcopyrite, and the grayish grains are sericite.
Simulated in situ leaching experiments were conducted in glass-lined steel pressure column reactors 30.5 cm (12 inches) in diameter and 3.05 m (10 feet) high. Figure 2 shows the reactor apparatus. Oxygen entered through the bottom and exited through a valve at the top. A titanium thermowell and solution sampling valve were also located at the top of the reactor. Vent gases were routed through 0.64-cm (0.25-inch) 316 stainless steel tubing to a pressure regulator and then at ambient pressure through a flowmeter. A needle valve in the line between the regulator and the flowmeter controlled the vent gas flowrate.
The shell of the reactor was wrapped with heating tape which was covered with 8.9-cm (3.5-inch) thick fiberglass insulation. The temperature of the ore-solution mixture within the reactor was adjusted with an electronic control which switched the heating tape on or off. Solution temperature was sensed with a type K thermocouple placed in the thermowell projecting 61 cm (24 inches) below the surface of the leach solution. Previous experiments had shown that this depth was sufficient to obtain a representative temperature of the contents of the reactor. The temperature was maintained at ±2° C of the operating temperature after the initial heat-up.
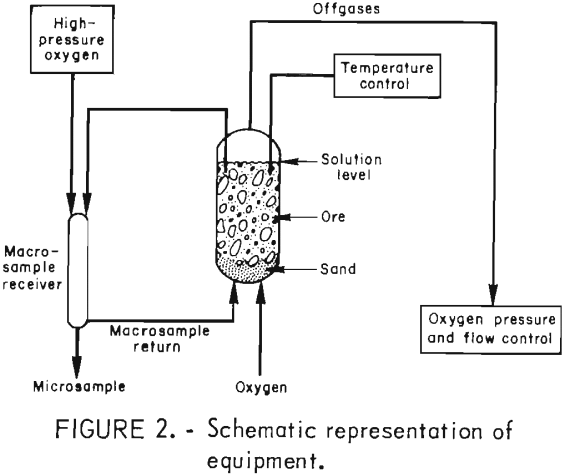
Accurate sampling required that solution samples of nearly 3 liters be withdrawn from the reactor. This size sample was representative of the liquid content within the reactor. Small samples were taken for chemical analysis and to determine the pH of the leach solution. After makeup acid and water were added to the remaining 3-liter sample, it was pumped into a 3,000-ml pressure cylinder and then injected into the bottom of the reactor through the oxygen supply line.
Experimental Procedure
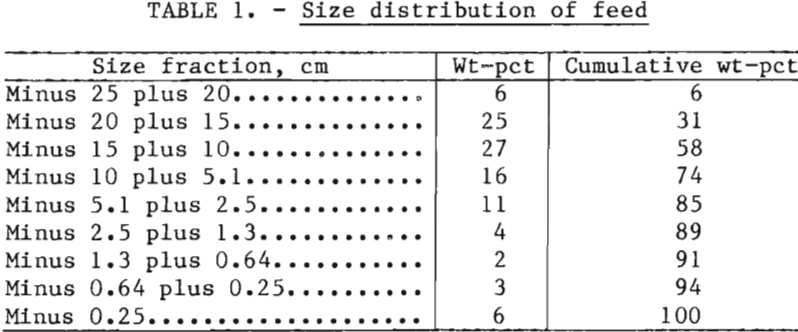
Two experiments, a baseline sulfuric acid leach and a sulfuric acid-NaCl leach, were conducted. The baseline experiment was conducted in a reactor of the same diameter but slightly taller than, the reactor used for the chloride leach. Consequently, the baseline charge was 167 kg (367 lb), whereas the chloride leach charge was 152 kg (333 lb). In either case, the size distribution of the charges was made up according to the schedule in table 1. Fragments larger than 10.2 cm (4 inches) comprised more than 50 pct of the weight of the charge, and the minus 0.25-cm (0.10-inch) ore made up 6 pct of the charge. Consequently, the majority of the ore contained sulfide mineralization that was not readily accessible to the leach solution because sulfides were encapsulated in the host rocks.
The ore was loaded into the top of the reactors by feeding portions of it into a measured amount of water. Some segregation of the fines occurred, but the fines would eventually migrate from the reactor bottom owing to solution currents caused by the oxygen bubbles rising through the column. The solution displaced by the ore was collected and carefully measured so that an accurate measure of the volumes of leach solution remaining in the reactors was obtained. The tops were bolted on the reactors, the contents were heated to the desired temperature, and then the solution was forced out of the sample dip tube until no more would come out. Finally. 3.0 liters of displaced solution was injected back into each reactor to complete the filling operation. This procedure also provided the 3-liter samples for chemical analysis. The final solution volume was 70 liters in each reactor.
The reactors were pressurized to 2.8 MPa (400 psig) oxygen pressure, and the oxygen was allowed to bubble through each reactor at a rate of 1.0 l/min. The bubbling oxygen insured that the leach solution was well mixed and oxygenated and that the solution temperature was uniform throughout the reactors.
The 3-liter solution samples were drawn in 14-day or longer increments. The longer sampling intervals were necessary because a minimum time of 2 weeks was required to uniformly distribute added reagents within the reactors. Each solution sample was analyzed for Cu, Fe, Na, K, Al, Mg, and Ca, and the pH was measured. Samples were periodically analyzed for Cl- and SO4²-. Residues were analyzed for Cu, Fe, Na, K, Al, Mg, Ca, S°, S²-, and SO4²-. Cu, Fe, Al, Mg, Na, K, and Ca concentrations were determined by atomic absorption spectrophotometry. Elemental sulfur, sulfate, sulfide sulfur, and chloride determinations were by wet-chemical methods. Solution pH values were made with an instrument having an accuracy of ±0.01 pH unit.
When the experiments were completed, the solutions were drained from the reactors and replaced with fresh water, which was allowed to digest with the ore for 2 weeks before the column was drained again. This procedure was repeated to assure that the leached ore was adequately washed. The leached residues were removed from the columns, dried, and screened through a series of screens down to 1 mesh, followed by wet screening of the undersize through 10 mesh. The size fractions were weighed, and representative samples of the large sizes of leached rocks were sectioned with a cut-off saw and examined for solution penetration and alteration. Mineralogical investigation of the leached rocks will be the subject of a subsequent report. Each unused half was returned to its size fraction, which was crushed and sampled. The head analyses used are calculated values based on the final solution and residue analyses and are corrected for the metal values lost during leaks and sampling throughout the experiment.
The 913-day, baseline experiment was conducted at 90° C and 2.8 MPa (400 psig) of oxygen pressure. The solution pH was decreased to and maintained at 1.90 for the first 440 days and then decreased to 1.75 for the remainder of the experiment. Concentrated sulfuric acid was used to maintain the pH of the leach solution.
Solvent extraction was used on the baseline leach solution on two separate occasions to decrease the copper content of the leach solution. In performing the solvent extraction, the leach solution was drained from the reactor, cooled, and treated in the conventional manner with a continuous countercurrent solvent extraction apparatus consisting of four extraction stages and three stripping stages. The extractant was a mixture of 12 vol- pct LIX 64N in kerosene. The pH of the raffinate was adjusted to the operating level with lime prior to reinjection of the solution. Details of the treatment are not reported here because the only purpose of the solvent extraction was to decrease the copper content of the leach solution. The first treatment decreased the copper concentration from 5.0 to 3.1 g/l; the second treatment decreased it from 3.9 to 1.8 g/l. The first and second treatments were made after 558 and 650 days, respectively.
The experiment evaluating the effect of NaCl on copper extraction was conducted for 1,236 days at 90° C and 2.8 MPa (400 psig) of oxygen pressure. The initial pH of the leach solution was 6.5. Acid was not added in the early stage of the experiment in order to determine if oxidation of the sulfides in the ore charge would provide sufficient acid to lower the pH to near 1.75 in a reasonable length of time. After 400 days, when it was apparent that the pH would not reach the target level, sulfuric acid additions were made to lower the pH and maintain it at 1.75. Salt addition was initially 2 wt-pct in solution. No further salt additions were made. The pH level of 1.75 was maintained for the next 600 days. The pH was finally decreased to 1.60 and was maintained at this level until the experiment was terminated. Solvent extraction was not used in this experiment because it was of interest to determine whether or not reactions would occur either in solution or with alteration products in the ore that would remove copper from the leach solution. Previous research employing a sulfate solution and the above test conditions on the ore showed no copper losses from solutions containing as much as 15 g Cu/l of leach solution.
Results and Discussion
Figure 3 compares the leaching results of the baseline-sulfate and the sulfate-containing-NaCl leach experiments. The pH profiles of the experiments are shown above the leaching curves. Figure 3A shows the actual solution copper concentration as a function of time, and figure 3B shows the percent copper extraction as a function of time. The curves of figure 3B are corrected for copper losses during sampling and from the few small leaks that occurred during the experiments. The data show that dissolution of copper was rapid during the initial phase of the baseline experiment and then became nearly constant. The extraction rate remained constant after the pH change but was at a slightly higher level. This continued until the experiment was terminated. When NaCl leaching was performed, there was no rapid initial rate of copper dissolution. A slowly decreasing extraction rate was observed until the pH was decreased to 1.75. The extraction rate increased rapidly to the approximate initial rate obtained in the baseline experiment and then decreased to the constant rate observed in the baseline experiment. When the pH was decreased to 1.6, the leaching rate again increased substantially.
Behavior such as that shown by the baseline experiment, where an initial
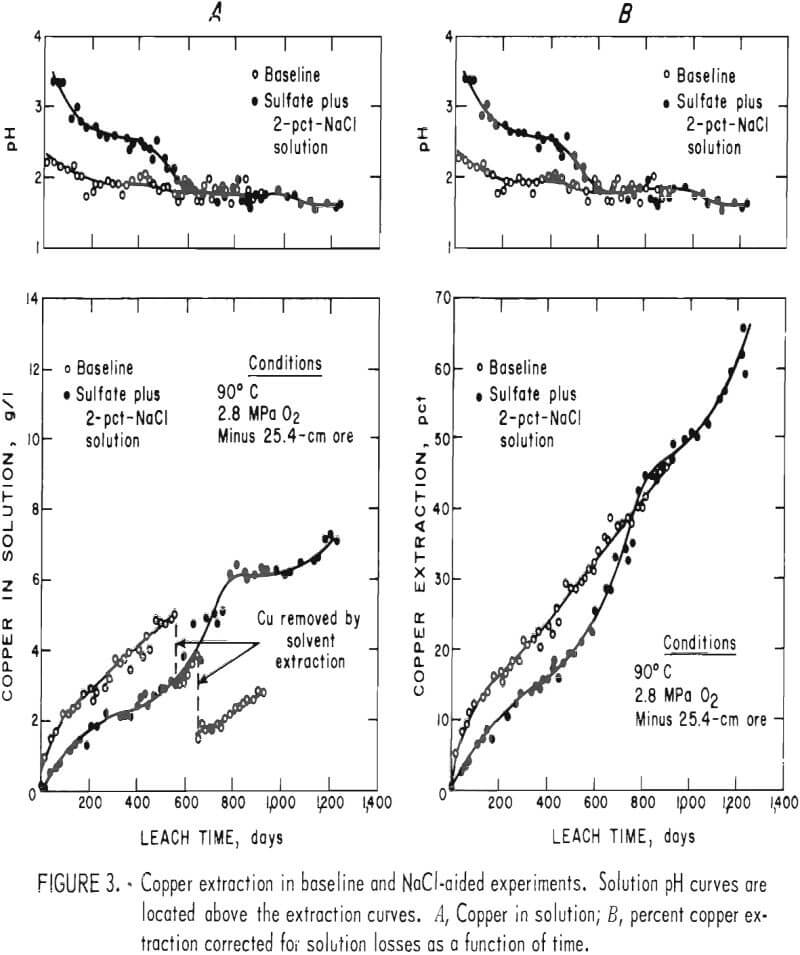
rapid but declining leaching rate is followed by a constant leaching rate, was discussed by previous researchers. The initial parabolic rate was the result of a rapid attack on surface or exposed copper minerals. The leaching rate decreases because the surface particles are dissolved and the solution must diffuse along cracks or through the host material and solid reaction products to continue copper extraction. The diffusion-controlled stage of leaching would be expected to produce a constantly declining extraction rate, in contrast to the constant leach rate that was observed in the second stage of the baseline experiment. This difference in observed versus predicted results can be explained by rock disintegration during leaching. Such behavior would reduce the diffusion path lengths in the ore fragments, thereby increasing the rate of mass transport (copper extraction) in the system.
Results observed in the NaCl-leach experiment indicate that a dynamic equilibrium was established between copper being dissolved from the sulfide minerals and copper being removed from solution by ion exchange with clay alteration products or by precipitation of basic copper sulfates or chlorides. This effect is pH dependent. Copper precipitation begins at very small solution concentrations when the pH is high, as shown by the portion of the curve up to 400 days of leaching. When the equilibrium of the system was changed by decreasing the pH, the leaching curve became similar to the initial part of the baseline experiment leaching curve, indicating that exposed copper, such as precipitates or copper adsorbed on clays was being leached. The increased dissolution rate continued until equilibrium was re-established at the new pH level.
The leaching data in figure 3A indicate that the equilibrium copper concentration in the NaCl-aided experiment was 6.1 g Cu/l at a pH of 1.75. At a pH of 1.60, the equilibrium conditions were not established, but the copper concentration would be more than 7.0 g Cu/l. These solution concentrations are considerably more than the normal concentrations of 1 to 2 g Cu/l that are obtained in current commercial dump or in situ leaching practice. In situ solutions from leaching of chalcopyrite ores would probably not contain higher copper concentrations because of slower extraction kinetics and the low grade of the ores being considered for leaching.
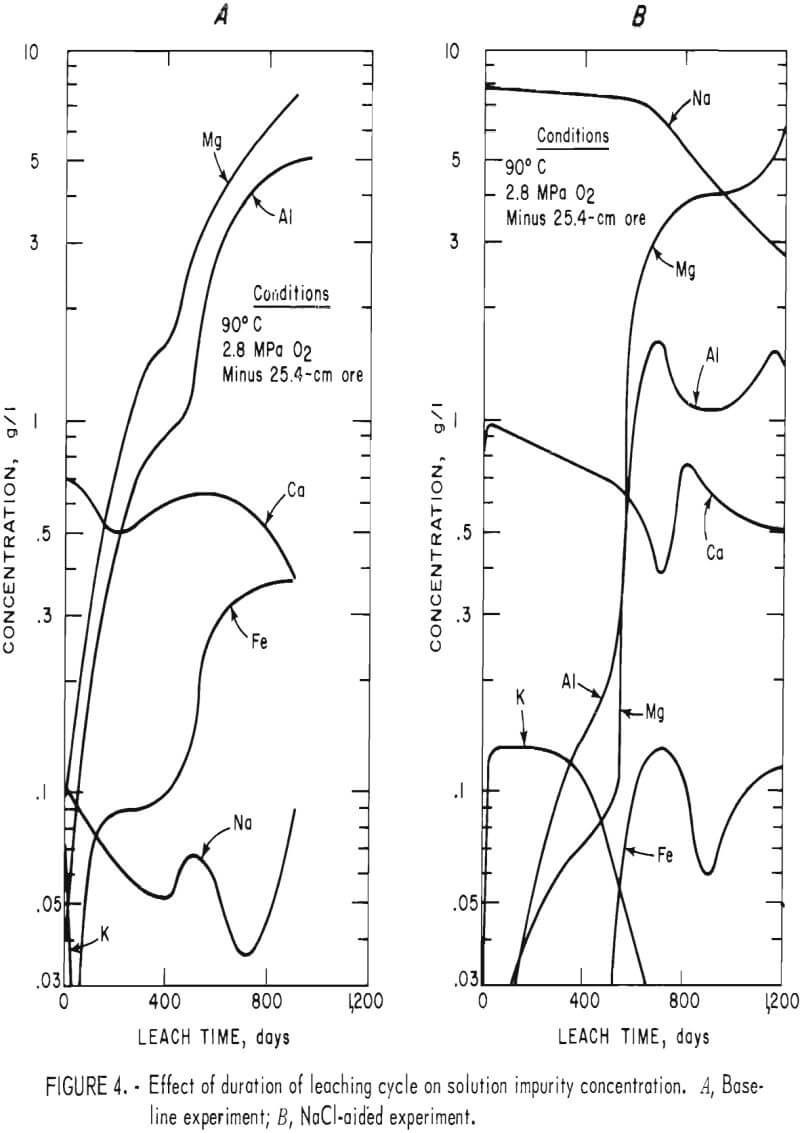
Impurities dissolved in the two experiments are shown in figure 4. Potassium and sodium solution concentrations in the baseline experiment increased rapidly. When sufficient iron and aluminum were dissolved to form jarosites and alunites, the potassium content became negligible and the sodium content decreased to less than 0.1 g Na/l. Iron content slowly increased to 0.09 to 0.1 g Fe/l when the solution had a pH of 1.95. When the pH was decreased to 1.7 5, the iron concentration increased to 0.37 g Fe/l. Magnesium and aluminum contents increased continually except when the pH was decreased from 1.90 to 1.75. After the third day of leaching, the calcium concentration remained reasonably constant because the calcium solubility limit was obtained. The solvent extraction treatments had no significant effect on the leaching solution impurity concentration.
The NaCl leach experiment produced similar trends for Mg, Ca, and K, whereas Al and Fe were affected by the high Na content of the leach solution. Both aluminum and magnesium concentrations increased markedly after the first pH adjustment, an indication that considerable reaction occurred between acid and gangue. After the pH level of 1.75 was established, the aluminum concentration remained fairly constant. Iron concentration was less than 0.15 g Fe/l throughout the experiment. Both iron and aluminum formed jarosite-type compounds. Calcium ion levels were similar to those obtained in the baseline experiment.
Chemical weathering and breakdown of the particles in a hydrometallurgical process are very important because the
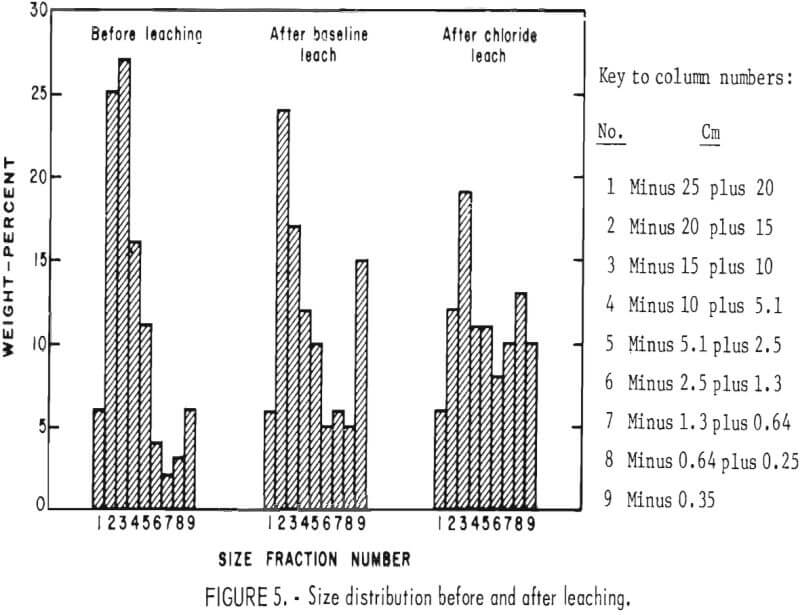
diffusion path for reactants and products through the rock is shortened; the end result can be a constant extraction rate, as was observed, or even an increasing extraction rate with time if significantly more disintegration occurred. Particle size distributions are shown in figure 5. The chloride leach experiment, which lasted 323 days longer than the baseline experiment, showed the greatest degradation In particle size. Because the leaching conditions in the two experiments were similar and fragmentation was greater in the experiment that lasted the longest, the conclusion can be made that particle fragmentation by weathering occurred throughout the experiments.
The analyses of the leach residues from the two experiments are shown in tables 2 and 3. Very little leaching occurred in the minus 25- plus 20.3-cm (minus 10- plus 8-inch) fraction. The copper content of the size fraction in both experiments was high after leaching because the particles had not degraded during leaching. As the particle size decreased, the amount of copper leached increased. The minus 1.3-cm (0.5-inch) fraction yielded almost all of its copper content in both leaching experiments.
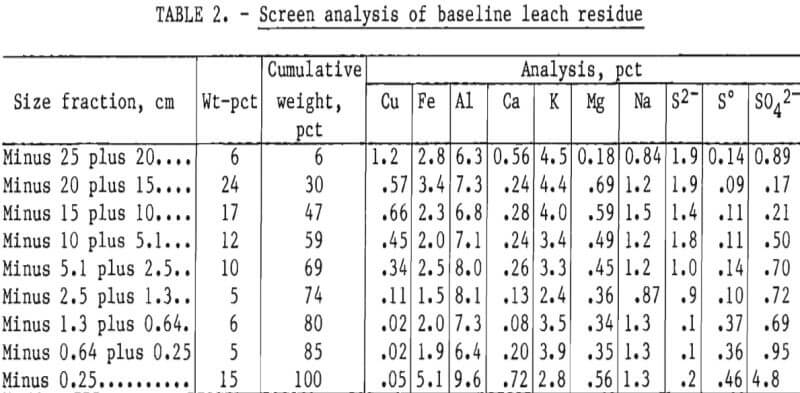
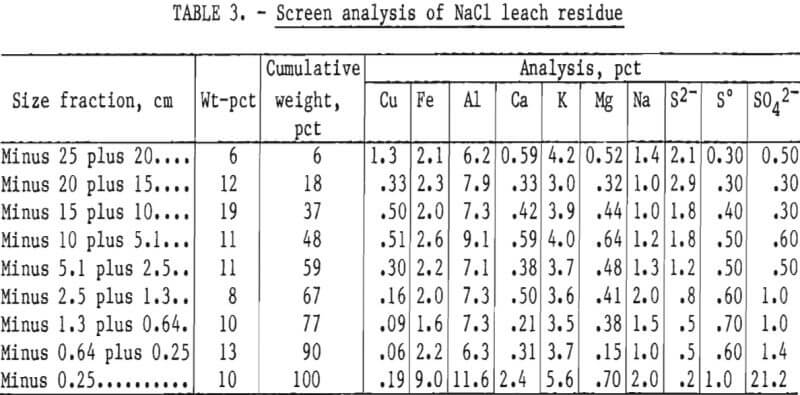
The iron content in all size fractions larger than 0.25 cm (0.10 inch) in diameter was similar. The high iron content of the minus 0.25-cm (0.10-inch) size fraction indicated that a jarosite precipitate was formed during leaching. The higher aluminum in the finest fraction of the residues indicated that alunite was formed. These compounds were confirmed by X-ray diffraction analysis. Magnesium was also enriched in the minus 0.25-cm fractions, especially in the NaCl leach residue, and may have partially substituted in the jarosite-type lattice, formed clays, and/or formed complex Mg-Fe sulfates or oxides.
Calcium content of the fine fraction was increased owing to precipitation of gypsum from solution. Sodium and potassium were not enriched in the fines of the baseline experiment. Hydrogen jarosite probably was the primary compound formed. Potassium and sodium contents were high in the fines obtained in the NaCl-leaching experiment. Sodium and potassium jarosite precipitates were probably formed in this experiment.
Sulfur and sulfate levels were high in the fines, but sulfide sulfur was low. These results indicate that most of the sulfide content of the feed was oxidized to sulfate and elemental sulfur during leaching, and that much of the sulfate precipitated as basic iron and aluminum sulfates. This was especially noticeable in the NaCl leaching system, where the dissolved aluminum, iron, and sulfate contents were considerably lower in all size fractions compared with the finest fraction.
Reagent consumption is another important consideration in any hydrometallurgical process. Oxygen and sulfuric acid are the major reagents that would require replenishment in the in situ leach system. Reagent requirements were computed on a per-kilogram-of-copper- extracted basis because the copper recovered must pay the expenses incurred during in situ leaching.
Oxygen consumption due to chemical reaction was deduced because most of the oxygen passed through the reactor and was unused. The deductive reasoning is based on the known reactants and their amounts, the observed products and their amounts, and evidence that most of the sulfide sulfur from the reacted pyrite is oxidized to sulfate under the conditions employed. The leaching reaction that, describes the observations in the baseline experiment involves converting CuFeS2 to CuSO4, jarosite, and elemental sulfur. These are the major products that were observed in the leach solution and residue. Although much of the iron was leached from the gangue minerals, the elemental sulfur contained in the leach residue was derived primarily from the chalcopyrite. Consequently, the amounts of elemental sulfur and copper that were solubilized indicate the predominant reaction that occurred during leaching. The amount of elemental sulfur contained in the baseline leach residue was 2 67 grams. The leach solution contained a total of 557 grams of copper. The mole ratio of sulfur to copper was
267/32.1 to 557/63.5 = 8.32 to 8.77
or nearly 1 to 1. The elemental sulfur produced in the chloride leaching experiment was 799 grams, and copper extracted was 831 grams. The mole ratio was
799/32.1 to 831/63.5 = 24.9 to 13.1
or nearly 2 to 1. Thus, leaching reactions were different in each experiment. The reactions, which are derived in the appendix, follow:
Baseline Experiment
6CuFeS2 + 6FeS2 + 39 O2 + 22H2O → 6CuSO4 + 4Fe3(SO4)2(OH)5 · 2H2O + 4H2SO4………………….(1)
NaCl-Aided Experiment
6CuFeS2 + 9FeS2 + 41.25 O2 + 5NaCl + 19.50H2O → 5CuSO4 + CuCl2 + 5NaFe3(SO4)2(OH)6 + 12S° + 3H2SO4 + 3HCl……………………………………(2)
The equations predict 39 and 41.25 moles of oxygen for every 6 moles of copper leached in the baseline and NaCl-aided experiments, respectively. The calculated oxygen consumptions were 3.27 and 3.46 kg/kg Cu extracted. The higher oxygen consumption in the NaCl-aided experiment was due to the larger amount of pyrite that reacted per unit of copper extracted.
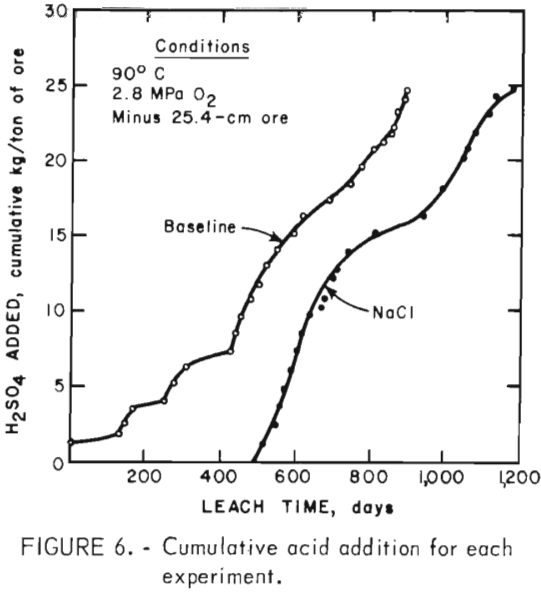
Sulfuric acid consumption in the baseline experiment was 4,094/557 = 7.35 kg/kg of copper extracted, whereas H2SO4 consumption in the NaCl experiment was 3,772/831 = 4.54 kg/kg of copper extracted. Figure 6 shows the acid added per ton of ore as a function of time. Acid was added earlier in the baseline experiment than in the NaCl experiment, but the acid was consumed at nearly the same rate. A relatively constant H2SO4 addition rate was necessary to maintain the pH at 1.80 in the baseline experiment, whereas the acid requirement decreased after 650 days in the NaCl experiment (pH of 1.75). Increased acid addition rates were required in the NaCl experiment when the pH was decreased to 1.60.
Conclusions
Experimental results showed that leaching minus 25.4-cm (10-inch) chalcopyrite ore with either dilute sulfuric acid or dilute sulfuric acid containing 2 pct NaCl under 2.8 MPa (400 psig) of bubbling oxygen and at 90° C yielded similar copper extractions. Diffusion of reactants and products into and out of the ore fragments controlled the leaching and prevented the more rapid copper leaching kinetics reported by other researchers who employed minus 1.27 -cm (0.50-inch) ore. The presence of NaCl promoted precipitation of copper minerals during leaching, especially when the pH was more than 1.75 and the copper concentration was more than 6 g Cu/l. However, precipitation was minimal at 1 to 2 g Cu/l, which is the expected concentration level in an in situ leaching system operated at pH 2.0. Iron and aluminum contents of the NaCl leaching solutions were substantially decreased owing to the formation of basic iron and aluminum sulfates. Magnesium, the other major solution impurity, was also precipitated. Calcium concentrations attained saturation after less than 3 days of leaching in each experiment. Diffusion into and out of the rock particles and a slow decrease in mean particle diameter due to weathering apparently controlled the leaching rate in each experiment. This precluded more rapid attack of the minerals by the chloride solution.
Calculated oxygen and measured H2SO4 consumptions for the two leaching systems were 3.27 and 7.35 kg/kg of copper extracted, respectively, for the sulfate and 3.46 and 4.54 kg/kg of copper extracted, respectively, for the NaCl- sulfate system. When a sulfuric acid solution containing NaCl is used for in situ leaching of a chalcopyrite ore, decreases in iron and aluminum impurity levels in the solution and in H2SO4 consumption were obtained. Increased corrosion of well casings, pumps, and surface facilities and the added expense of chloride additive must be balanced against decreased impurity level and acid consumption.
