Table of Contents
Chromium is essential to modern industry, and there are few acceptable substitutes for most of its metallurgical and chemical applications. In 1979 the United States consumed 610,000 tons of chromium, of which about 10 pct was supplied from secondary sources from within the country; the remainder was imported. Annual consumption is projected to grow to 1.24 million tons by 2000. All large, high-grade deposits of chromite, the major mineral source for chromium, are located in the Eastern Hemisphere; only Cuba and Brazil in the Western Hemisphere have significant reserves of minable ore. Recent chromite imports have come primarily from South Africa, the U.S.S.R., Turkey, and Zimbabwe. Because of this dependence on foreign sources of chromium, the Bureau of Mines has had a continuing interest in identifying domestic resources and developing technology for their utilization.
Domestic chromium resources for which proven recovery technology exists are of three types. These resources are located in the Pacific Coast States and Montana and contain collectively 1.75 million tons of chromium. One type is the podiform deposits located in Oregon and California; there primary ore bodies are usually small, scattered, and high grade. Another primary type is represented by the large, low-grade, high-iron content stratiform deposit located in the Stillwater (Montana) complex. The secondary black sand placer deposits along the southern Oregon coast also contain low-grade, high-iron chromite. Except for a small quantity of chromite that was produced and exported in 1976, there has been no domestic mining or beneficiation of chromite ore since 1961 when Federal purchasing programs ended.
In the past the Bureau developed beneficiation procedures for domestic chromite materials using methods that included flotation, gravity concentration, and magnetic and electrostatic separation. Recently a flotation procedure was developed for beneficiating Stillwater chromite ore, and a gravity and magnetic separation system was developed for beneficiating low-grade California ores.
Although very low grade, the laterites of southwestern Oregon and northern California represent the largest secondary occurrence of chromium in the United States and therefore attract attention as a source for chromium. These laterites are soils remaining after decomposition of ultrabasic rock and contain major quantities of Co and Ni in addition to Cr. The Bureau has developed a hydrometallurgical process to recover the nickel and cobalt from laterite that includes reduction roasting, ammoniacal sulfate leaching, solvent extraction, and electrowinning. This process yields a residue that contains the chromite in an unaltered form. The recovery of chromite from either the leach residue or the unprocessed laterite was investigated and is the subject of this report. The use of this residue as a chromite source provides economic advantages in that it is already mined, milled, and slurried for beneficiation.
Other investigators have proposed methods for simultaneous recovery of Ni, Co, and Cr from lateritic ores. Takahashi applied ore-dressing procedures to prepare separate nickel- nonmagnetic iron, cobalt, magnetic iron, and chromite concentrates. Harvey proposed a pressure, hydrochloric acid leach procedure to extract Ni, Co, Cr, and Fe, followed by precipitation of the Fe. In the Bureau approach conventional ore-dressing methods were used to recover chromium either before or after hydrometallurgical extraction of nickel and cobalt.
Laterite Resources
Laterite Geology and Mineralogy
Peridotite rocks, altering to serpentine, predominate over much of the Klamath Mountains vicinity of southwestern Oregon and northern California. These rocks were chemically weathered to laterites during the Tertiary tropical environment and the subsequent temperate environments. The olivine and pyroxene of the peridotite decomposed, and the calcium, magnesium, and silica constituents were leached away, leaving a soil enriched in nickel, cobalt, and iron, the less soluble constituents. Chromite and magnetite present in the original rock resisted weathering and are residual minerals in the partially altered saprolitic and more altered lateritic soils. Soil depth varies, but it is thicker on ridge tops, benches, and other areas of gentle slopes.
The predominant mineral in lateritic soils is very fine goethite, FeO(OH), which contains most of the nickel. A wadlike manganese, oxide contains the cobalt. Laterite mineralogy varies widely and typically contains massive and fibrous serpentine, chlorite, quartz, talc, hematite, tremolite, and enstatite. The residual primary minerals include magnetite and chromite.
The laterites examined during this investigation contained 25.0 to 33.0 pct Fe, 0.7 to 1.1 pct Ni, 0.06 to 0.10 pct Co, 1.3 to 1.9 pct Cr, 9.6 to 13.2 pct MgO, and 14.9 to 27.6 pct SiO2.
Extent of Resources
The Bureau of Mines is evaluating the chromium-bearing nickeliferous laterites of southwestern Oregon and northern California. The size of the resource will be determined through a combination of seismic studies and auger and channel sampling. Data compiled through 1979 infer a resource exceeding 157 million dry metric tons. Three-quarters of this is in Del Norte County, Calif.; the balance is in Josephine and Curry Counties, Oreg.
Leach Residue Mineralogy
The Bureau of Mines has developed a hydrometallurgical process to recover nickel and cobalt from lateritic ores. The nickel and cobalt are selectively reduced and then extracted by an oxidizing ammonia-ammonium sulfate leach. During reduction the goethite and hematite are converted to magnetite, metallic iron, and taenite (ferronickel alloy). Partial reoxidation of these iron constituents occurs during leaching. Essentially all of the iron in the laterite leach residue is ferromagnetic, though much of it is too fine to respond well to low-intensity magnetic separation. Except for partial iron reduction and extraction of nickel and cobalt, there is little change in laterite mineralogy resulting from treatment by the Bureau process.
Character of Resources
Laterites from typical domestic deposits were sampled and wet sieved. Analyses of the samples are shown in table 1. The subsieve fractions were further sized on a Warman Cyclosizer, a series of hydraulic cyclone elutriators that continues the standard screen series down to 11 µm. Laterites were treated at 10 kg/hr in the Bureau’s reduction roast-ammoniacal leach process research unit, which continuously extracted nickel and cobalt. Samples of the extraction residues were sized. Weight and constituent distributions of the laterites and leach residues are shown in figures 1 to 4. The curve discontinuities are transitions between screen and sedimentation sizing.
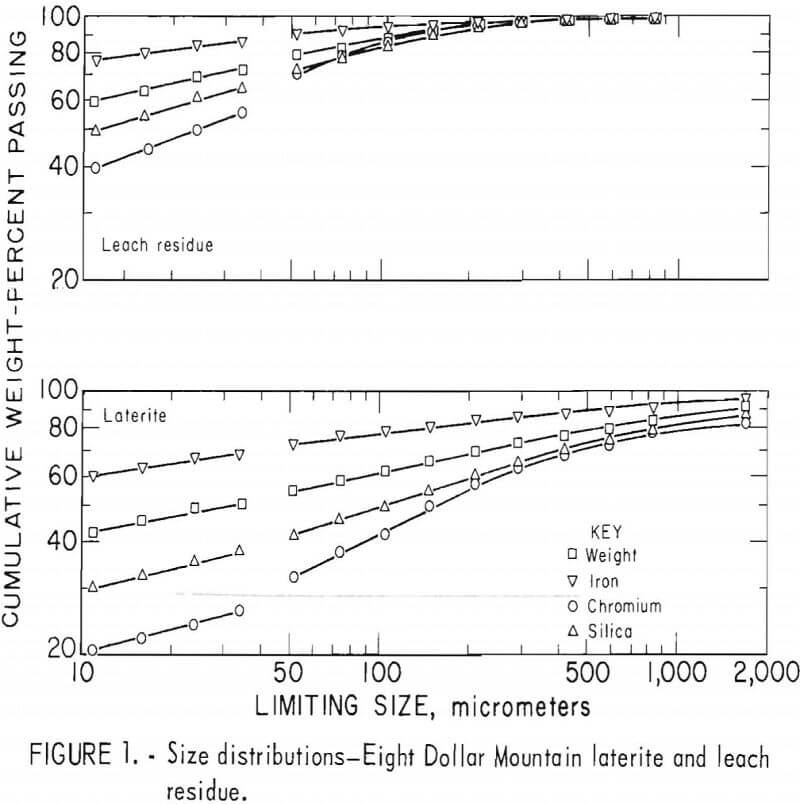
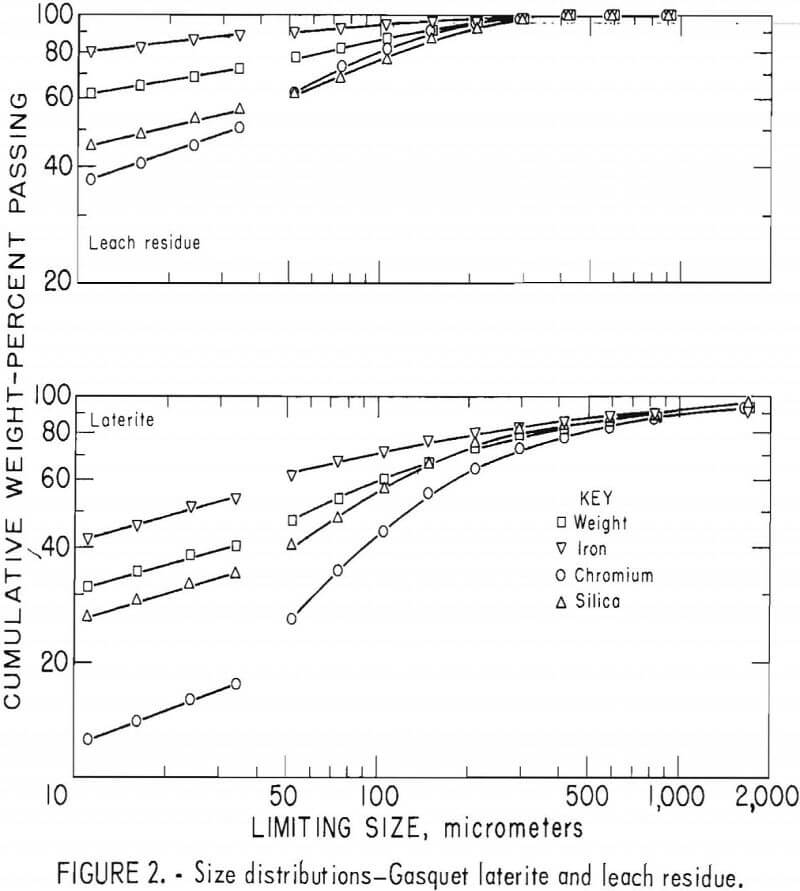
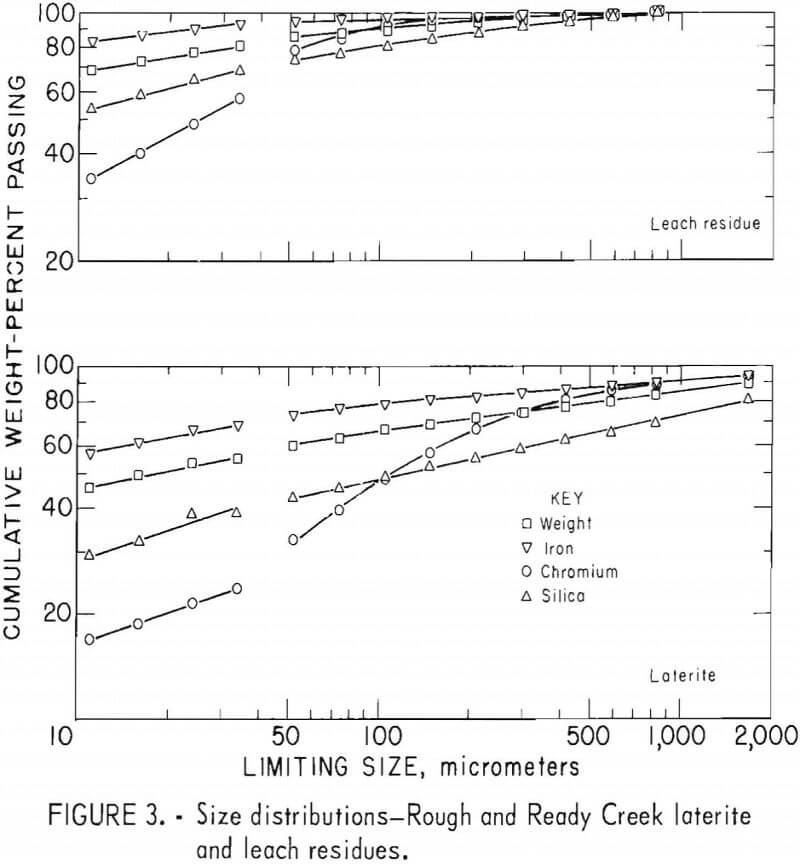
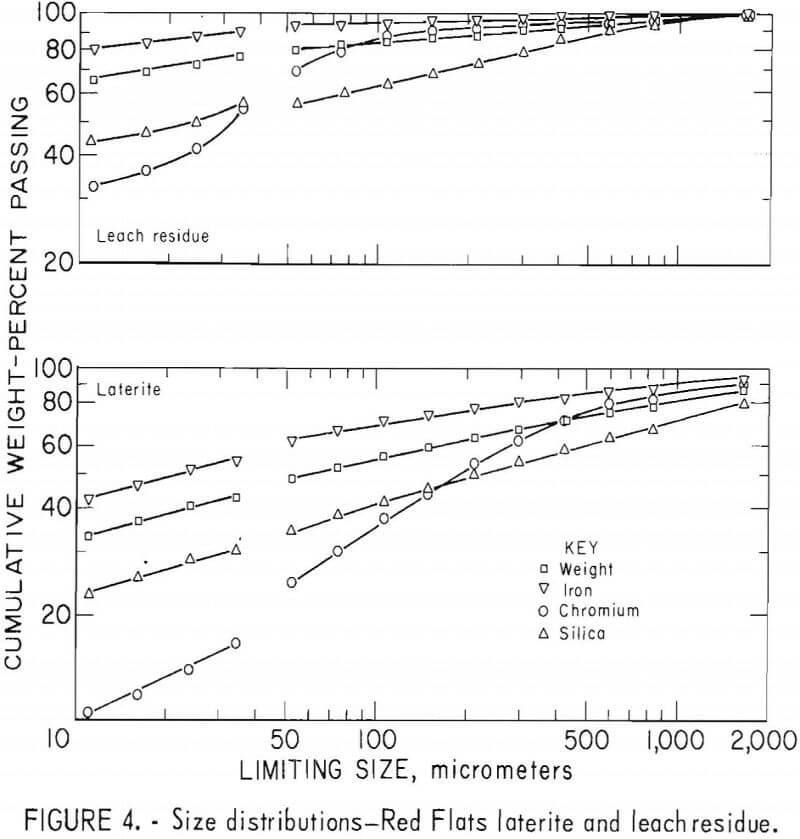
Between 40 to 55 pct of the laterites and 60 to 80 pct of the leach residues are finer than 37 µm (minus 400 mesh). Up to 46 pct of laterites and 70 pct of residues are finer than 11 µm. The finest fraction of each is predominantly iron oxide, present as goethite in the laterite and as reduced goethite in the residue. The coarsest constituent of each residue, coarser than 50 µm, is serpentine (as reflected by silica content).
Chromite tends to concentrate in the intermediate-sized fractions, although it is finer in the residue than in the laterite. The minus 11-µm fractions contain 10 to 20 pct of the chromium in the laterites and 32 to 40 pct of the chromium in the residues. The fine size of the residue and its chromite resulted from comminution in the Bureau’s nickel-cobalt process research unit.
Samples of laterite and residue were further sized with a Micromeritics Sedigraph, a sedimentation-rate sizing instrument. Leach residues contained 65 to 83 pct minus 10-µm and 8 to 26 pct minus 1-µm particles and had an average particle equivalent diameter (d50) of 2.2 to 3.4 µm. Laterites contained about 11 pct minus 1-µm chromite and had an average particle diameter of 8 µm.
Chromite Identification
A high-purity chromite concentrate was isolated by repeated high-intensity magnetic and electrostatic separations performed on a sample of Pine Flat laterite. X-ray diffraction analysis of the concentrate showed that it contained a single spinel with a unit cell of 8.24 A. The crystallographic data and chemical analysis of the concentrate indicate this spinel to have the composition Mg0.45Fe0.55(Cr0.65Al0.30Fe0.05)2O4. A pure concentrate of this chromite would contain 34.6 pct Cr (50.5 pct Cr2O3) and have a Cr-Fe ratio of 1.86:1.
Another high-purity chromite concentrate was prepared from Rough and Ready Creek leach residue by multiple magnetic and gravity separations. The concentrate contained 31.0 pct Cr (45.3 pct Cr2O3), 15.9 pct Fe, 11.0 pct MgO, 9.8 pct Al2O3, and 0.36 pct SiO2. The chromite in this concentrate is similar to that from the Pine Flat laterite. It is shown in figure 5 along with elemental X-ray maps that illustrate the substitution of magnesium for ferrous iron and the substitution of alumina for chromia in individual mineral grains.

Beneficiation
Chromite Recovery From Laterite Leach Residue
Although chromite in residues is finer than that which is considered to be efficiently recoverable by conventional ore-dressing techniques, these techniques seem most appropriate for low-grade materials. A simple, straightforward recovery system was sought. Dry processing methods such as electrostatic and high-intensity magnetic separation were tried and found to inadequately recover the chromite. Flotation and other procedures requiring reagent additions were not successful in preliminary beneficiation tests. All of these methods were inadequate because of the high slime content of the residue.
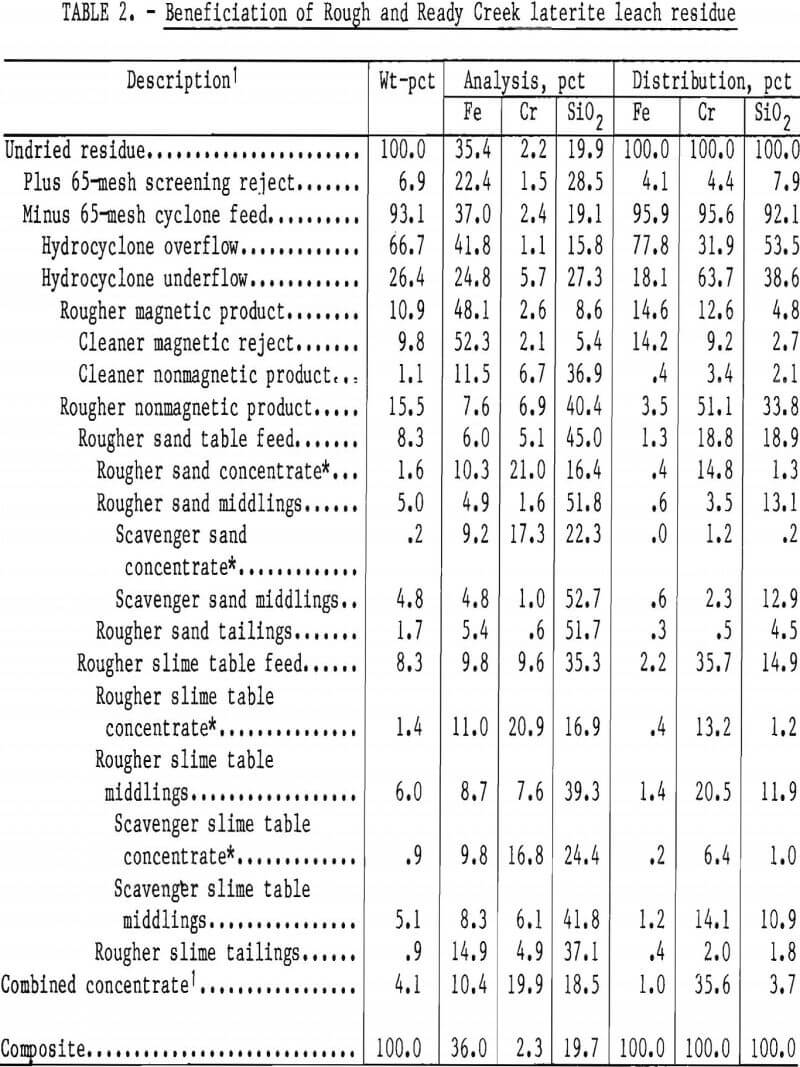
The best beneficiation approach was to take early advantage of differences in size distributions of the predominant iron slimes and the chromite. The procedure shown in figure 6 was applied to Rough and Ready Creek leach residue. Residue was first attritioned at 50 pct solids to break up agglomerates. Screening at 65 mesh rejected very little chromite with a coarse serpentine product. Single-pass desliming of the screen undersize fraction with a 25-mm hydrocyclone rejected most of the iron into a fine (90 pct minus 10 µm) overflow product, while most of the chromium was retained in the coarser underflow fraction. Wet low-intensity magnetic separation of the cyclone underflow removed residual magnetite along with some of the reduced goethite. The nonmagnetic product was screened at 200 mesh to divide it into approximately equal parts to enhance tabling. Two stages of sand tabling were performed on the screen oversize; two stages of slime tabling were performed on the undersize product.
Results of beneficiation of Rough and Ready Creek leach residue are summarized in table 2. A concentrate containing 19.9 pct Cr (29.1 pct Cr2O3) and having a Cr-Fe ratio of 1.9:1 was prepared from residue containing 2.3 pct Cr (3.4 pct Cr2O3). The high silica analysis (18.5 pct) of the combined concentrate reflects the ferromagnesian silicate mineral content. That and the low chromium recovery, 36 pct, are the result of fine particle size rather than insufficient mineral liberation. The largest chromium loss was to the cyclone overflow, but this chromite was finer than 10 µm and was unseparable from the iron slimes by usual ore dressing methods. Chromium losses to magnetic products were unavoidable because of the tendency of magnetic particles to agglomerate and occlude chromite. A single retreatment of the rougher magnetic product included high-frequency, high-intensity demagnetization of the pulp before reintroduction to the separator. Magnetic cleaning provided nearly 7 pct of the chromite in the table feed.
Chromite Scavenging by Flotation or High-Intensity Wet Magnetic Separation
A substantial amount of the chromite was lost to slime table middlings. Microscopic examination of this product revealed essentially only two constituents—small angular chromite fragments and larger blocky crystals of enstatite. Attempts were made to scavenge chromite from table rejects by sizing with screens or hydrocyclones, but this provided neither satisfactory grade nor adequate recovery. Because of the fine sizes, poor separations were also made with electrostatic-electrodynamic methods.
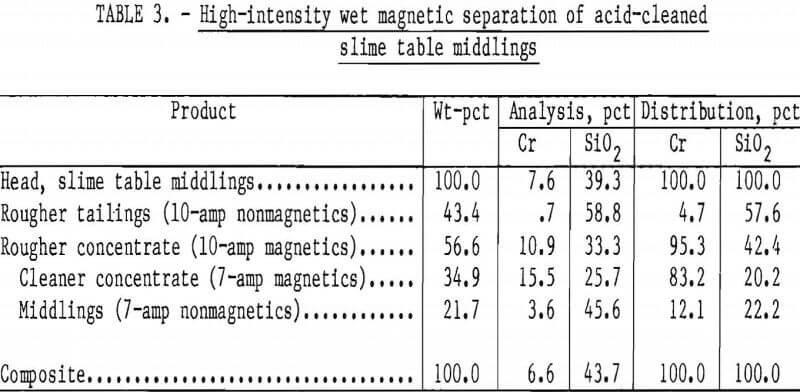
Preliminary tests using flotation or high-intensity magnetic separation were unsuccessful in recovering chromite from table middlings. Overlapping magnetic susceptibilities were found to result from nearly invisible iron oxide surface films on the mineral particles. Scrubbing in a 15-pct solution of hydrochloric acid removed these coatings and rendered samples responsive to high-intensity magnetic separation. The acid-cleaned chromite had a magnetic susceptibility greater than 21.4×10 -6 emu/g, as determined by a method developed by Hopstock. The cleaned enstatite showed susceptibilities between 8.6 and 13.7×10 -6 emu/g. These differences indicated potential for successful high-intensity magnetic separation. Other acids and lower concentrations of hydrochloric acid were less effective cleaners of mineral surfaces and did not enable effective separation.
A representative sample of slime table middlings from the beneficiation procedure shown in figure 6 and summarized in table 2 was leached briefly in 15-pct hydrochloric acid. The removal of the surface films consumed 110 g HCl per kilogram of table middlings. The cleaned middlings were treated in two stages with a high-intensity separator having an expanded iron matrix. Results are summarized in table 3. The rougher separation, performed at 10-amp field current and 9,100-gauss airgap magnetic intensity, rejected a final tailings comprising 43 pct of the total weight and containing 5 pct of the total chromium.
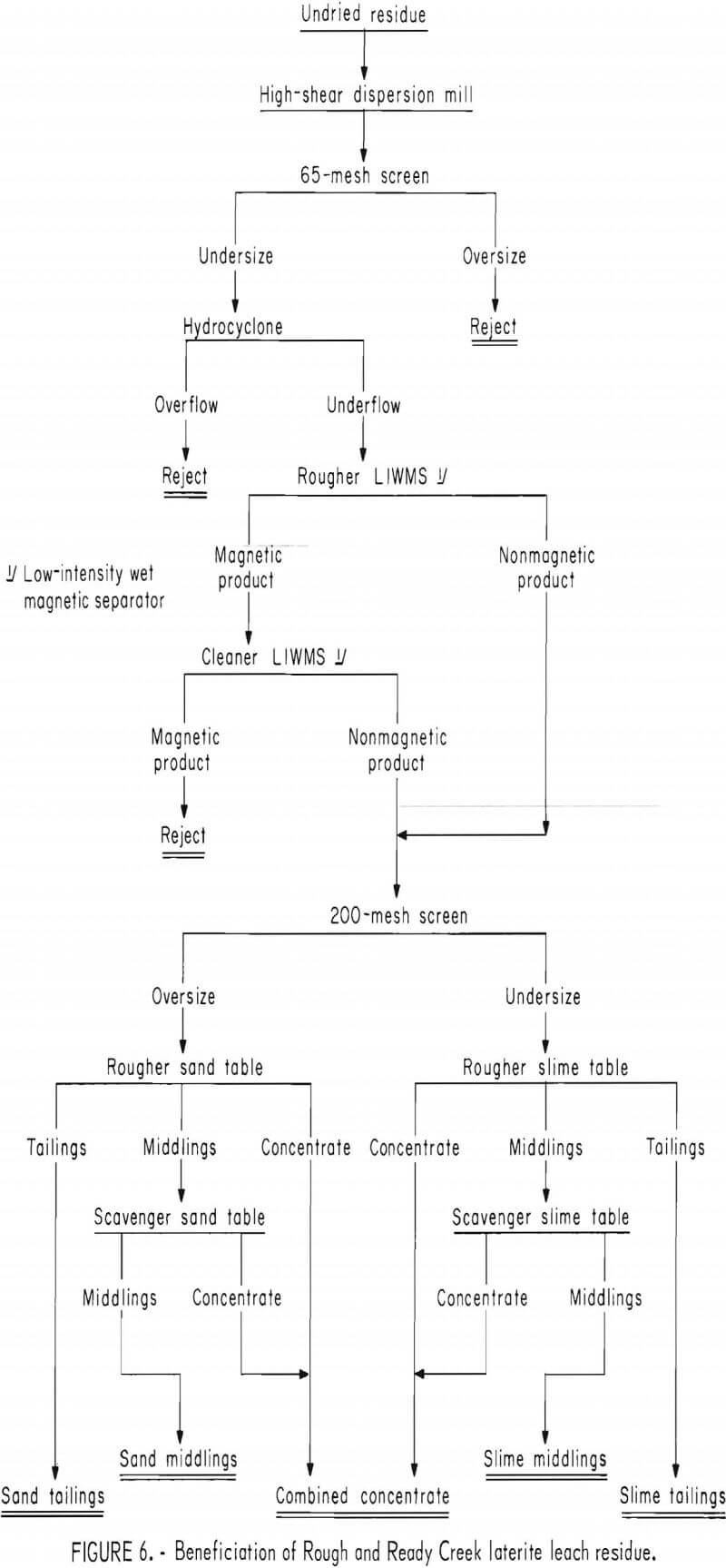
The rougher concentrate was retreated at 7 amp, 7,800 gauss to give a middling product and a cleaner concentrate containing 15.5 pct Cr (22.7 pct Cr2O3) at a chromium recovery of 83 pct. The middlings contained 12 pct of the chromium and would be recycled to rougher separation or additional acid scrubbing in continuous beneficiation. High intensity magnetic scavenging of chromite from slime table middlings increased the overall chromium recovery from Rough and Ready Creek laterite residue to 47 pct as a concentrate containing 18.6 pct Cr, 10.0 pct Fe, and 20.8 pct SiO2.
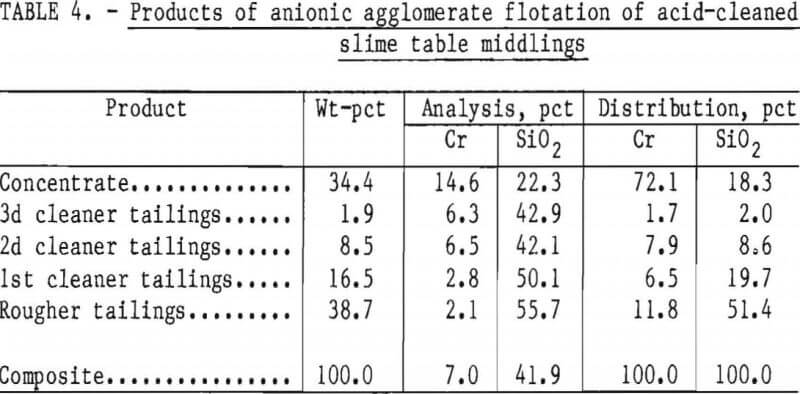
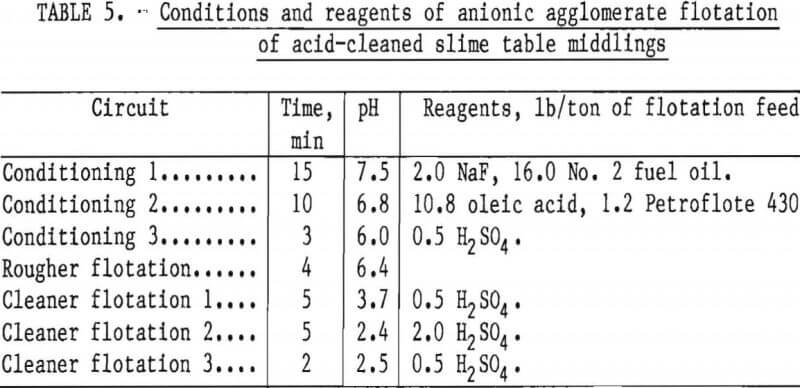
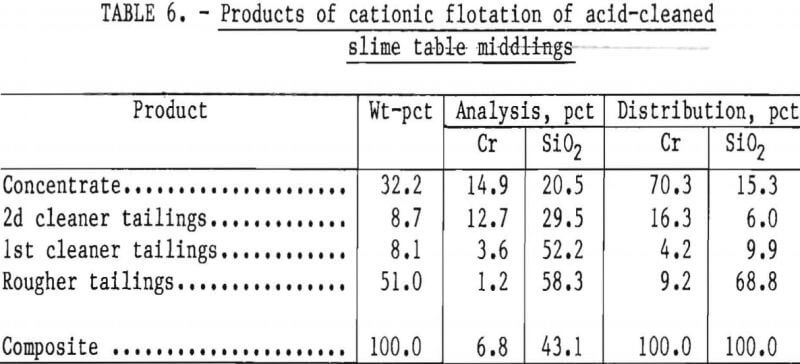
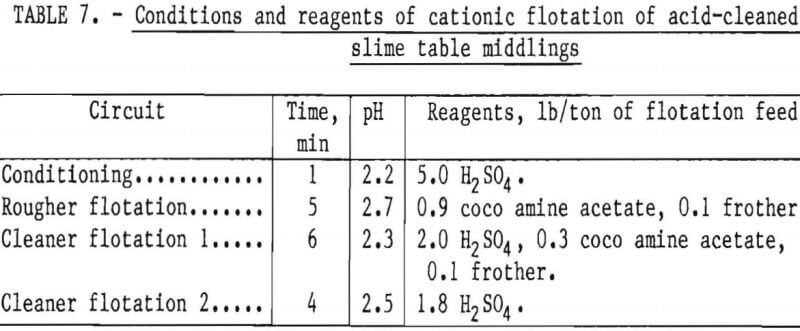
Two flotation methods also were used to successfully scavenge chromite from acid-scrubbed slime table middlings. The first of these was anionic agglomerate flotation by the method described by Hunter the procedure and results are summarized in tables 4 and 5. Large reagent additions were necessary to recover 72 pct of the chromium in a triple-cleaned concentrate that still contained 22 pct SiO2. In the second method, a cationic flotation procedure developed by Smith, less reagent was required. The application of this procedure is summarized in tables 6 and 7. Very similar results were obtained by both flotation methods and magnetic separation. All procedures required thorough preliminary mineral cleaning with hydrochloric acid. The added chromium recovery probably does not justify the large acid requirement.
Chromite Scavenging by Gravity Separation
A Bartles-Mozley laboratory vanner, shown in figure 7, was used to simulate two industrial gravity separators, the Bartles-Mozley concentrator and the Bartles crossbelt concentrator, in treating residue slime table rejects. The vanner and the two industrial machines use thin water films and orbital shear to recover heavy minerals from slime (5- to 100-µm) feeds. The Bartles-Mozley concentrator is essentially a gravity pre-concentrator designed to reject a clean tailings and achieve moderate concentration. The Bartles crossbelt concentrator achieves substantial enrichment but produces a tailings that should be retreated on the preconcentrator. These machines are frequently used in tandem; each is simulated by the vanner by adjusting its slope.
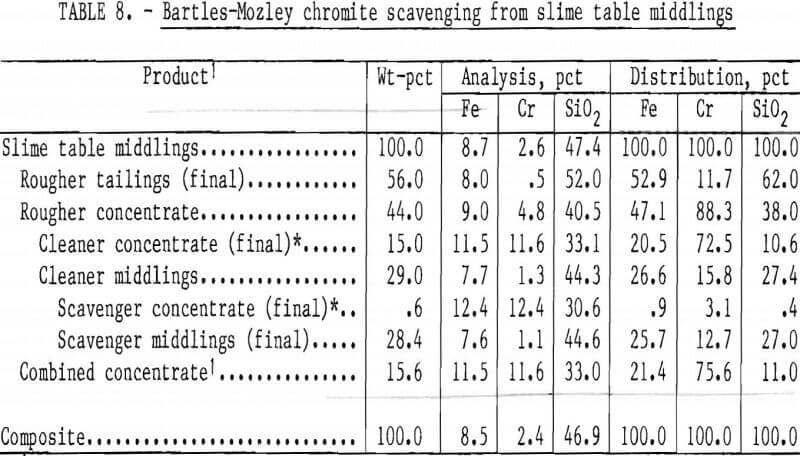
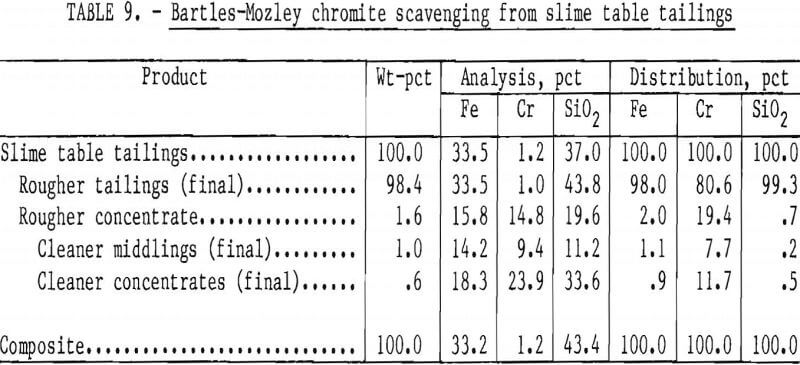
The Bartles-Mozley vanner was used to treat slime table middlings and tailings from Gasquet leach residue. This residue contained 1.6 pct Cr, and its beneficiation by the procedure shown in figure 6 resulted in a recovery of only 29 pct of the chromium as a concentrate containing 20.9 pct Cr (30.5 pct Cr2O3). Both slime tabling products were given an initial scavenger treatment simulating the Bartles-Mozley concentrator and a subsequent cleaner treatment simulating the crossbelt concentrator. The cleaner middlings were recleaned on the simulated crossbelt. Results, shown in tables 8 and 9, were promising; recoveries and grades were comparable to those obtained by flotation or magnetic separation with acid scrubbing. A scavenger concentrate containing 23.9 pct Cr (34.9 pct Cr2O3) was recovered from table tailings containing only 1.2 pct Cr. The combination of Bartles-Mozley-scavenged concentrates with the primary gravity concentrates resulted in an overall chromium recovery of 35 pct as a product containing 18.0 pct Cr (26.3 pct Cr2O3), 12.5 pct Fe, and 21.9 pct SiO2.
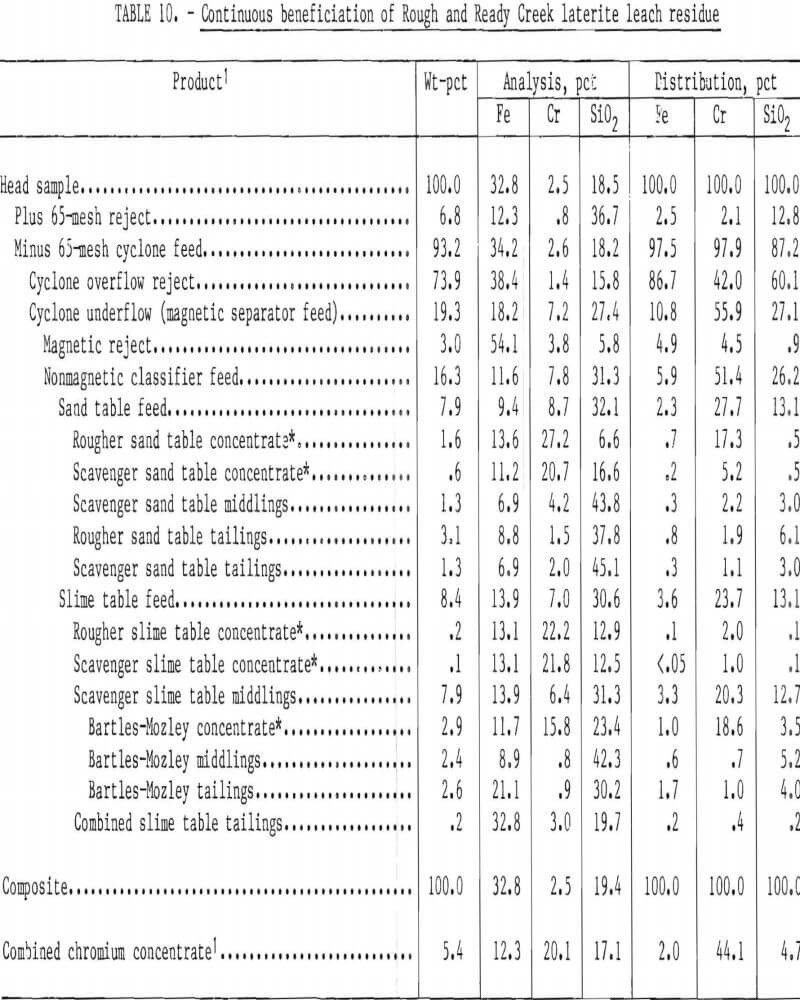
Bartles-Mozley scavenging of table middlings was included in a continuous beneficiation scheme for Rough and Ready Creek residue. Preliminary beneficiation was by the procedure shown in figure 6, except that nonmagnetic table feed was hydraulically classified rather than screened. Classification was in an elutriator at a waterflow rate resulting in a separation at approximately 200 mesh. Hydraulic classification directs more chromite to the sand table than screening. Beneficiation results are summarized in table 10. Chromium recovery was 44 pct as a concentrate containing 20.1 pct Cr (29.4 pct Cr2O5) with a 1.6:1 Cr-Fe ratio while rejecting 98 pct of the Fe and 95 pct of the silica. Gravity scavenging of slime table middlings yielded 42 pct of the total chromium recovered.
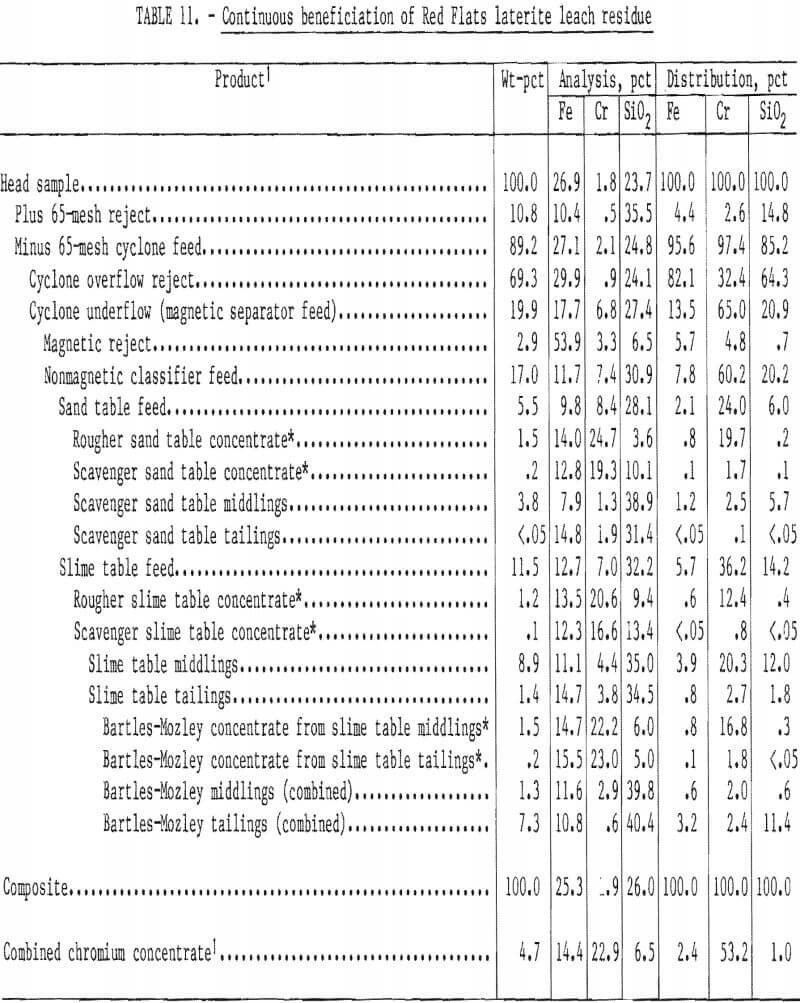
A lower grade and finer residue from Red Flats laterite was processed similarly. Beneficiation results are summarized in table 11. Overall recovery was 53 pct as a concentrate containing 22.9 pct Cr (33.4 pct Cr2O3) with a 1.6:1 Cr-Fe ratio. In this test Bartles-Mozley scavenging was applied to both middlings and tailings from rougher slime tabling. About 33 pct of the total chromite recovery was obtained from the middlings and 2 pct from the tailings. These results appear comparable to those achieved by acid scrubbing and high-intensity magnetic separation. Gravity scavenging avoids the use of reagents and their subsequent recovery, removal, or neutralization.
Chromite Recovery from Laterite
The procedure for recovery of chromite from leach residues, described in the previous section, was applied to a sample of Rough and Ready Creek laterite—the source of residue for the beneficiation test summarized in table 10. Initial sizing was at 20 mesh because of the coarser size of the laterite. The plus 20-mesh product was rejected although it contained nearly 15 pct of the chromium. The 20- by 65-mesh fraction was separated magnetically and table-concentrated. The minus 65-mesh fraction was treated exactly like the residue derived from this laterite (fig. 6). Beneficiation results are summarized in table 12. The beneficiation procedure was as effective with laterite as with residue, although more coarse chromite should have been recovered. Forty percent of the chromium was recovered as a concentrate containing 17.4 pct Cr (25.4 pct Cr2O3) with a Cr-Fe ratio of 1.4:1. The nickel and cobalt in the laterite reported primarily to seven sizing and tabling products that contained collectively 82 pct of the nickel and 84 pct of the cobalt.

Utilization of Chromite Products
The character of chromite concentrates from laterites and laterite leach residues suggests they would be most suitable for use in the manufacture of chromium chemicals. The chromite is very fine, is low grade, and has a low Cr-Fe ratio. However. concentrates have a higher silica content than is generally used for chemical-grade chromite applications.
Preliminary experiments using a procedure developed by Smellie and Branslatter, 90 pct of the chromium in an unground slime table concentrate to be solubilized and extracted in a three- stage hot water leach. The concentrate had been blended with soda ash and ground limestone and roasted in air at 900° C prior to leaching. Neither silica nor iron was extracted by this method; aluminum extraction ranged from 30 to 56 pct.
Selective reduction roasting followed by acid leaching of the reduced iron increased Cr-Fe ratios of lateritic chromite concentrates to as high as 4:1. However, removal of part of the iron without removal of the predominant impurities does not permit significant increase in the chromium content.
Research on the utilization of the chromite products is continuing.
Iron Coproduct Recovery
Nickel-cobalt laterites and laterite leach residues contain up to 45 pct iron. An effort was made to recover this constituent as a separate product. The iron is predominantly in two forms. The first is relatively coarse magnetite that is a residual mineral in the laterite and is unchanged by the reduction roast- ammoniacal leach treatment; the second is the goethite. The magnetite is 2 to 8 pct of the total weight and contains up to 15 pct of the total iron. It was recovered as a low-grade magnetic product containing 47 to 51 pct iron by low- intensity magnetic separation with a concurrent drum. Some nonmagnetic minerals, including chromite, report to the magnetic product occluded with magnetic aggregates. The rougher magnetic product was too low grade to be of interest as an iron product and represented a significant chromium loss. The rougher magnetic slurries were pumped through a high-frequency, high-intensity demagnetizing coil before cleaning with the drum separator. Cleaned magnetic concentrates contained up to 58 pct Fe and as little as 1.8 pct Cr and 4.7 pct SiO2; however, alumina and magnesia were high. Single-stage cleaning resulted in 72-pct recovery of the iron in the rougher magnetic product, and the clean concentrate contained as much as 13 pct of the total iron in the residue. Magnetic recleaning increased the grade above 58 pct Fe but decreased the recovery even more. Gravity separation and flotation were less effective than magnetic separation in cleaning magnetic products.
Goethite is the principal constituent of laterite, making up about one-half of its weight. In treating the laterite by reduction roasting and ammoniacal leaching, the goethite is reduced to maghemite or a hydrated magnetite. Both goethite and reduced goethite are very fine, and most of it reports to the cyclone overflow in the beneficiation procedure. The cyclone overflow slimes from beneficiation of a typical laterite residue (Red Flats) had an average particle diameter (d50) of 2.0 µm. Nearly a quarter of the slimes was finer than 1.0 µm. The cyclone slimes analyzed 30 to 40 pct Fe and represented 80 pct of the iron in the laterite or residue. Goethite or reduced goethite in the hydrocyclone overflow did not respond satisfactorily to beneficiation efforts because of its fine size and the presence of contaminating surface films on non-ferrous mineral particles. Anionic flotation with fatty acids or petroleum sulfonates, cationic flotation with primary amines, and gravity concentration were all ineffective. Multiple treatments with a concurrent drum magnetic separator increased grade from 40-45 to 45-49 pct Fe at an 18-pct recovery. Each pass decreased recovery because each nonmagnetic product contained nearly as much iron as the magnetic products. Acid scrubbing to remove surface coatings before magnetic separation improved the grade up to 57 pct Fe, but this resulted in dissolution of much of the iron.
Summary and Conclusions
Laterite and the leach residue remaining after extraction of cobalt and nickel from laterite were processed to recover chromite. At the onset of the study, it was expected that the fine size of the chromite would preclude its ready response to established mineral dressing techniques. Research led to the development of a processing procedure that allowed recovery of up to 53 pct of the chromium values in the laterite as a concentrate containing 22.9 pct Cr (33.4 pct Cr2O3) with a 1.6:1 Cr-Fe ratio.
The processing scheme that was devised takes advantage of several physical characteristics of the laterite materials. Because the major constituents have different size distributions, significant waste can be separated by sieving and hydrocyclone sizing. An intermediate-sized product containing the bulk of the chromite is then processed by low-intensity magnetic separation to remove ferromagnetic iron oxides, leaving a mixture made up predominately of chromite and enstatite. The coarser fraction of this mixture responds well to gravity concentration—specifically sand tabling. The finer fraction responds less efficiently to slime tabling. A concentrated research effort was made to perfect a method for recovering the chromite from this fine fraction.
Initial efforts to recover the fine chromite lost to slime table rejects were unsuccessful. Nearly invisible ferromagnetic iron oxide films coated mineral particles and prevented their separation. It was found, however, that agitation leaching of the material in acid removed the films and enabled scavenging the fine chromite by either high-intensity magnetic separation or flotation. Either method allowed chromium recoveries of about 50 pct, but the cost of the acid consumed in removing the oxide films exceeded the value of the additional chromite recovered.
As a potential lower cost alternative, equipment designed specifically for gravity concentration of heavy minerals from slime-sized feeds was tested. Equipment simulating a Bartles-Mozley preconcentrator and a Bartles crossbelt concentrator was used to successfully scavenge the fine chromite from slime table middlings without precleaning particle surfaces of iron oxide. Overall chromium recoveries with this final processing scheme were similar to those obtained using the acid treatment; that is, about 50 pct.
The magnesia, alumina, and iron contents of the chromite associated with domestic laterite limit the attainable product grade. In addition, the fine particle size of much of the chromite limits recovery of the mineral, the grade, and the ultimate use of the concentrate. The chromite has neither the size distribution nor the composition preferred for refractory or metallurgical applications. Its best use would appear to be in the manufacture of chromium chemicals, where fine size would be advantageous in promoting digestion by alkali. However, additional research would be necessary to develop this digestion technology.
The potential recovery of high-grade iron oxide byproducts from laterite residue was also investigated. Acceptable concentrates could not be prepared from either the major goethite or the minor magnetic constituents of the residue. The best concentrates produced by low-intensity magnetic separation suffered from high alumina and magnesia content and represented only a small fraction of the iron oxide present in the material (less than 15 pct of total iron recovery).
It can be concluded from the investigation that—
- The extremely fine size character of the chromite in lateritic materials will limit its recovery to about 50 pct of the contained chromium by conventional physical separation methods.
- Chromite concentrates produced from the southwestern Oregon-northern California laterites will be limited to 25 pct Cr (36.5 pct Cr2O3) with a Cr-Fe ratio of 1.6:1 at a chromium recovery of 50 pct. If lower recoveries were acceptable, concentrates containing as high as 31 pct Cr (45.3 pct Cr2O3) with Cr-Fe ratios of 1.9:1 could be prepared.
- With a beneficiation procedure including only sizing and magnetic, and gravity separation procedures and no chemical reagent additions, there would be minimal environmental impact associated with the chromite recovery.
- Although iron oxides are the predominant constituents of the laterites, concentrates of marketable quality could not be produced with satisfactory recovery.
