In situ leaching has been an attractive means of recovering uranium from small or low grade ore deposits. The process involves injecting a lixiviant, typically ammonium carbonate-bicarbonate and an oxidant into the uranium containing ore body. As the solution passes through the ore body, the uranium is oxidized from the insoluble plus four to the soluble plus six state. The uranium is then completed with the anion of the lixiviant (e.g., carbonate-bicarbonate). The uranium laden solution is removed from the ore body and the uranium recovered in an above ground processing plant.
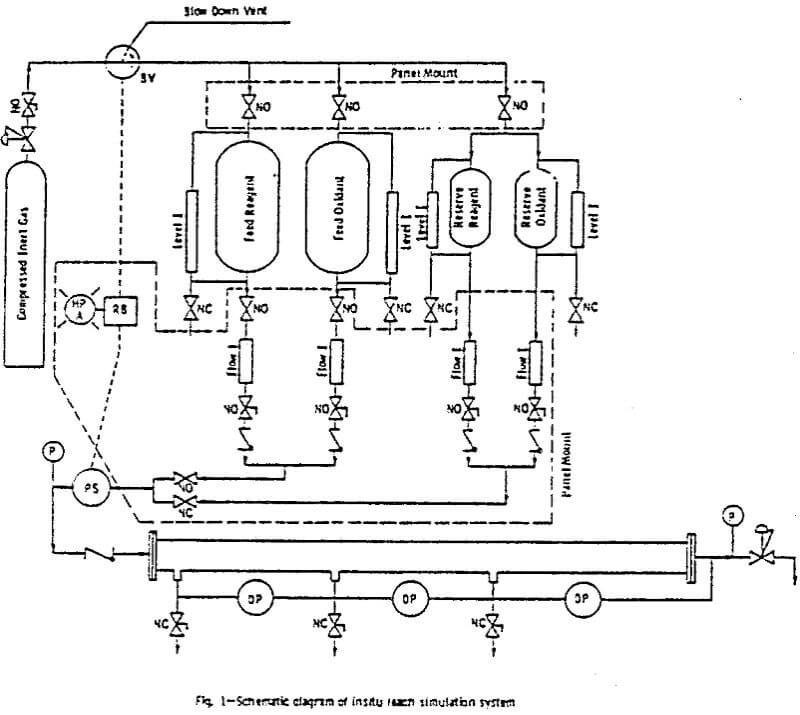
The system contains pressurized feed tanks, from which the leach solution is fed into the ore column. The feed system consists of two primary 316 stainless steel 0.06 m³ (lb gal) tanks, having an allowable working pressure of 830 kPa (100 psig). One tank contained a PVC liner which served to separate the pressurizing gas (nitrogen) from solutions (deionized water and ground water), in which gas saturation was not desirable.
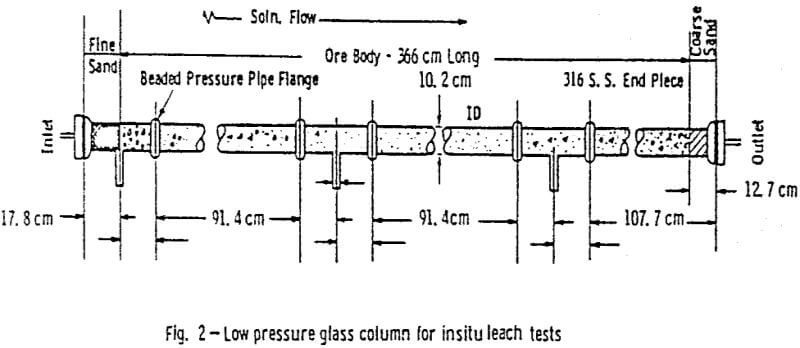
The ore used in this study was obtained from the Lucky Mc mine in the Shirley Basin of Wyoming. The entire batch of ore was air dried, crushed and ground to -10 mesh, and then blended together. Preparation of the ore in this manner seemed to facilitate packing and insured the uniformity of samples used in the tests.
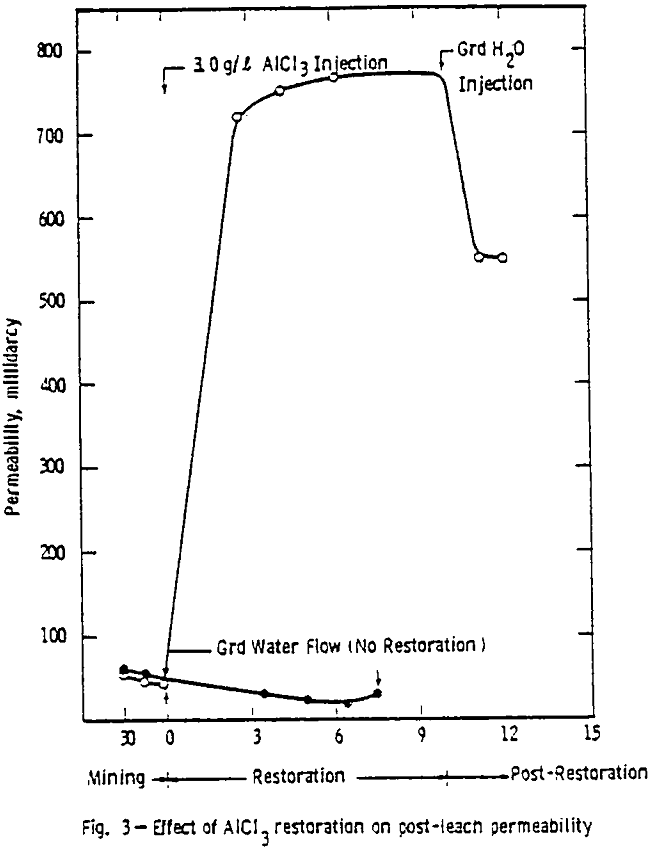
After the permeability and stability of the packing was found acceptable, the 1.0 g/l ammonium bicarbonate leach solution saturated with oxygen at 345 kPa (50 psig) was introduced. A packing was acceptable if the permeability was within the range considered proper for this ore (0.6 to 2 µm³) and if no cracks or ore compaction occurred.
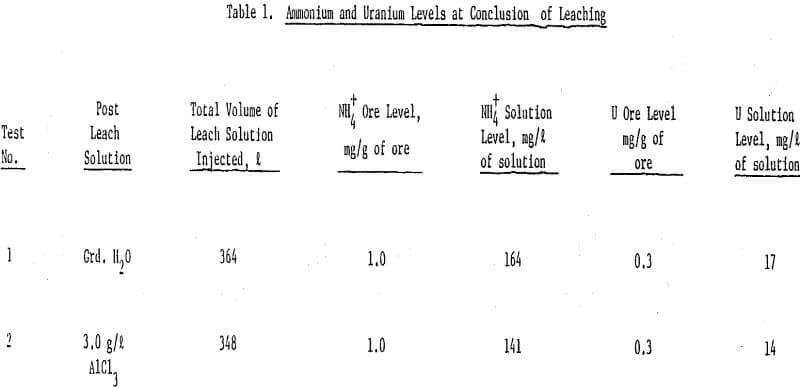
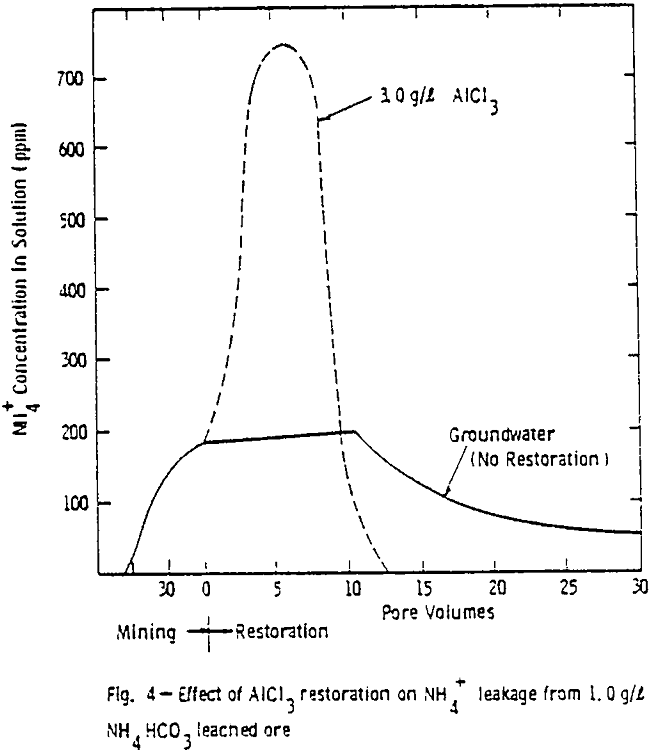
The results show that at the conclusion of leaching the ammonium sorption characteristics and uranium levels were essentially the same for both columns of ore. That is, at the end of ammonium bicarbonate leaching, the ammonium concentration in the ore was 1.0 mg NH4+/g of ore and a uranium level of 0.3 mg/g of ore for both ore columns.
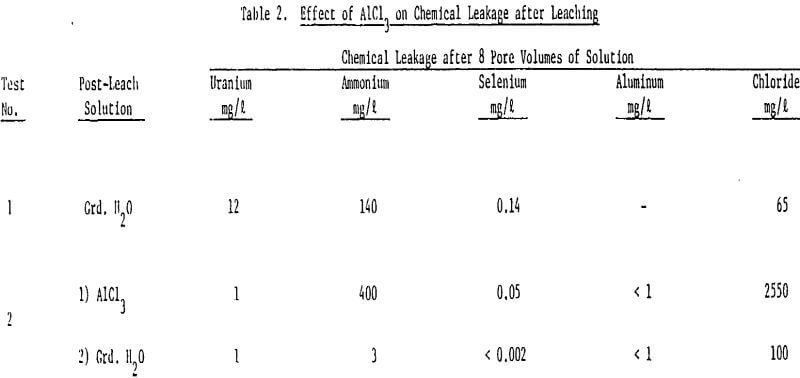
The large decline in ammonium leakage with aluminum chloride restoration is the direct result of the high ammonium removal achieved during the aluminum chloride flow. Over 90% of the ammonium on the ore was removed in 10 pore volumes of the aluminum chloride. The trivalent aluminum resulted in rapid ammonium removal. Little ammonium was thus left to desorb into subsequent ground water flow.