Experiments were conducted with the addition of several types of flocculants and surfactants. The filtration response of the slurries was studied in terms of the cake formation rate and the residual cake moisture. An analysis of the results is presented in ascertaining the role of flocculants and surfactants during filtration
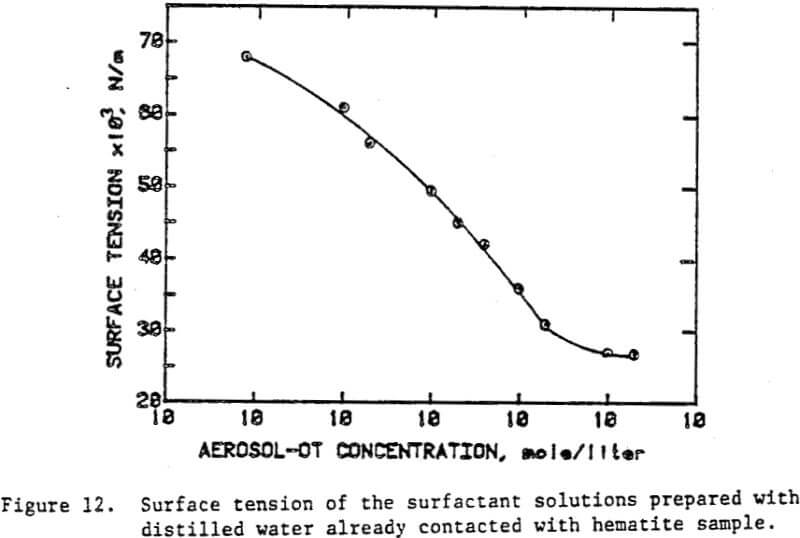
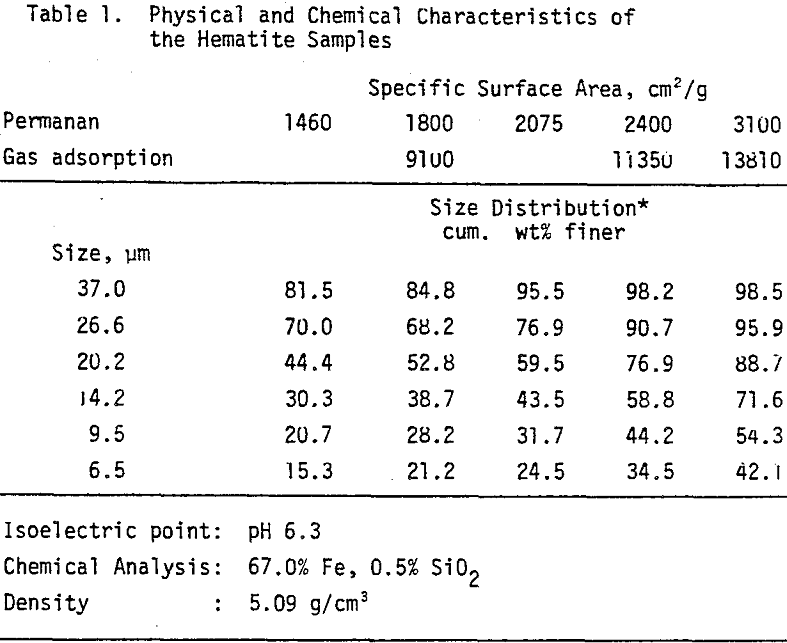
A specular hematite concentrate (67% Fe, 0.5% SiO2) wet ground to desired fineness was used as a standard sample in all the experiments. The isoelectric point of the sample was found to be at pH 6.3 by conducting electrophoretic measurements.
Test slurry samples were prepared by dispersing the required amount of moist solids in distilled water and then adding the flocculant and surfactant while mixing. The slurry was poured into the filter unit and the desired vacuum was applied. The time period required for water to disappear from the cake surface was recorded as form time. The vacuum application was continued until the cake dewatering ceased. Cake thickness was measured and a round sample was cut out by means of a thin-walled pipe for moisture determination. Cake moisture is reported on wet weight basis.
Experimental Results and Discussion
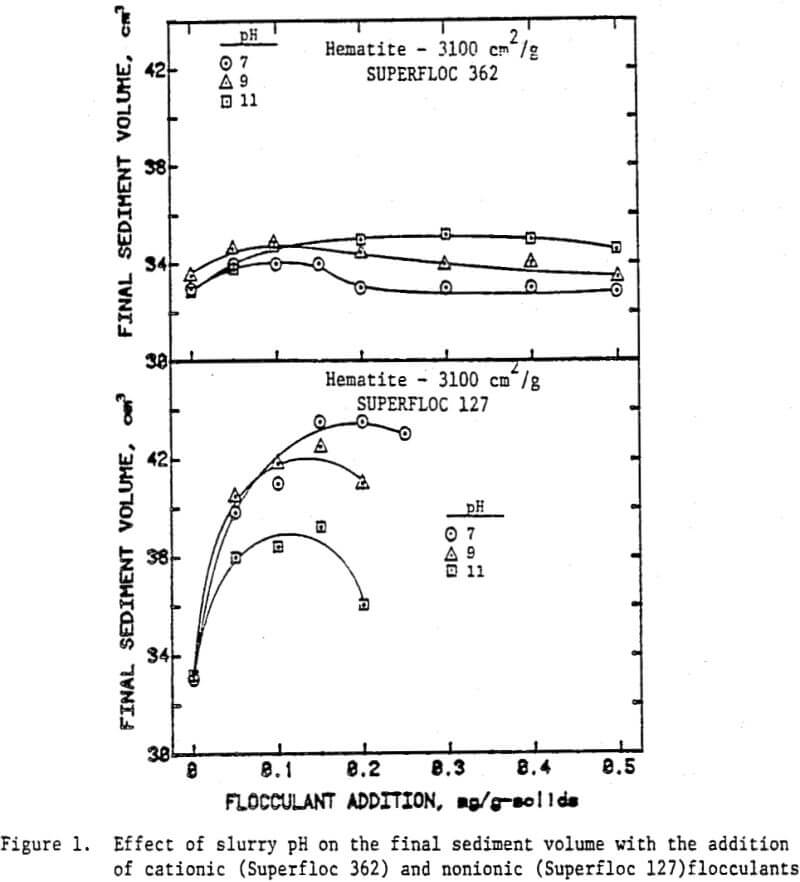
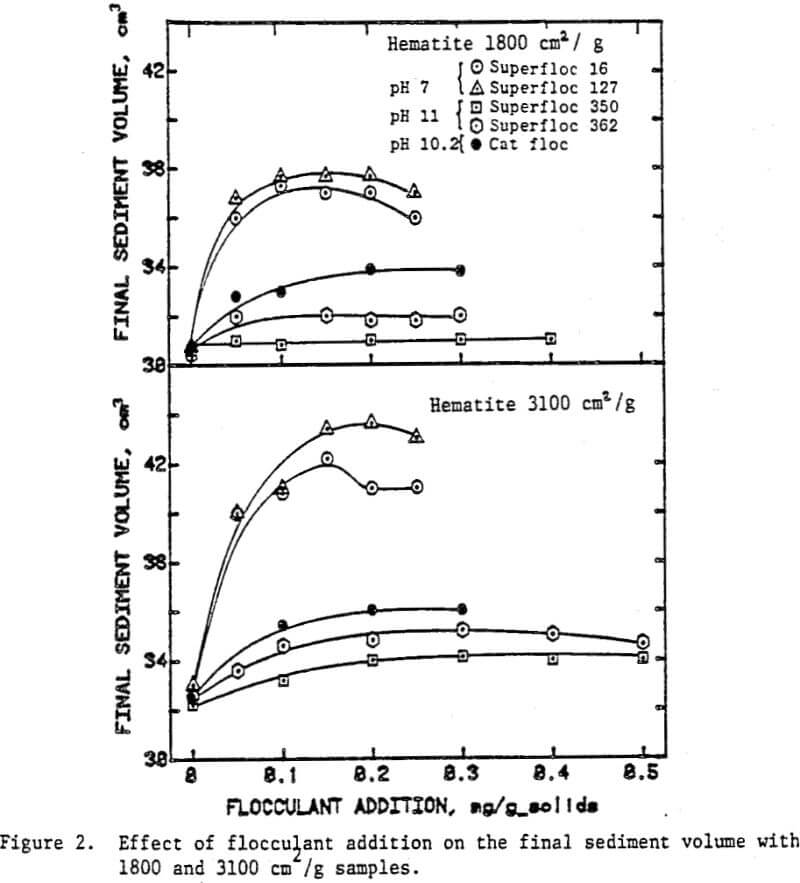
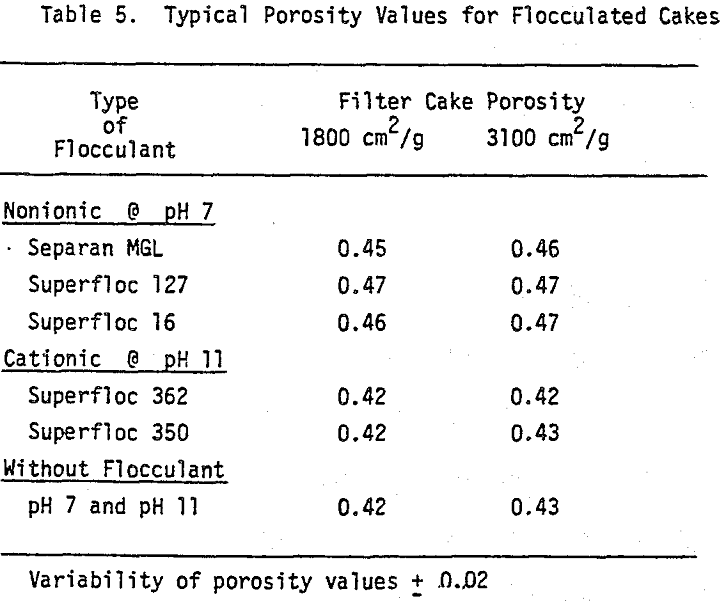
The volume of the sediment at the completion of settling gives a measure of the extent of flocculation and the nature of flocs as well. High sediment volumes generally correspond to large floes and loose floe structures. Among the factors determining the floc size and the density are the type of flocculant added, slurry pH and agitation intensity. Figure 1 shows the effect of slurry pH on the final sediment volume as a function of the added dosages of the cationic and the nonionic flocculants.
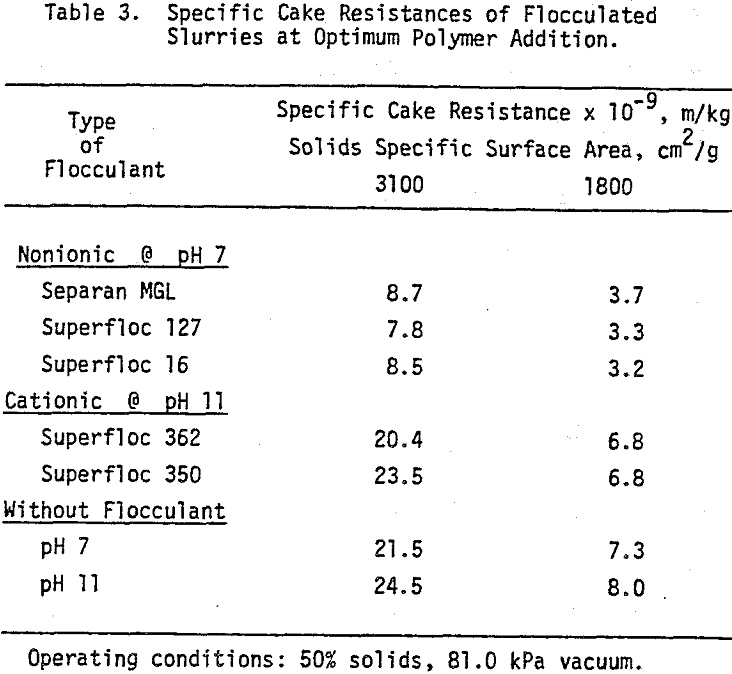
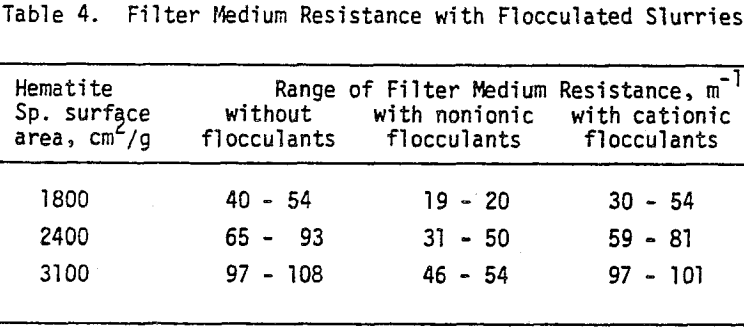
The filter medium resistance amounted to about 20 percent of the final cake resistance. If we consider the fact that the filter medium resistance is expected to remain essentially unchanged after the initial stages of filtration, higher pressure losses across the medium should be observed at the initial and intermediate stages where higher filtrate flow rates are involved as compared to the final moments of cake formation. Therefore, neglecting medium resistance for theoretical and practical purposes will not always be justifiable.
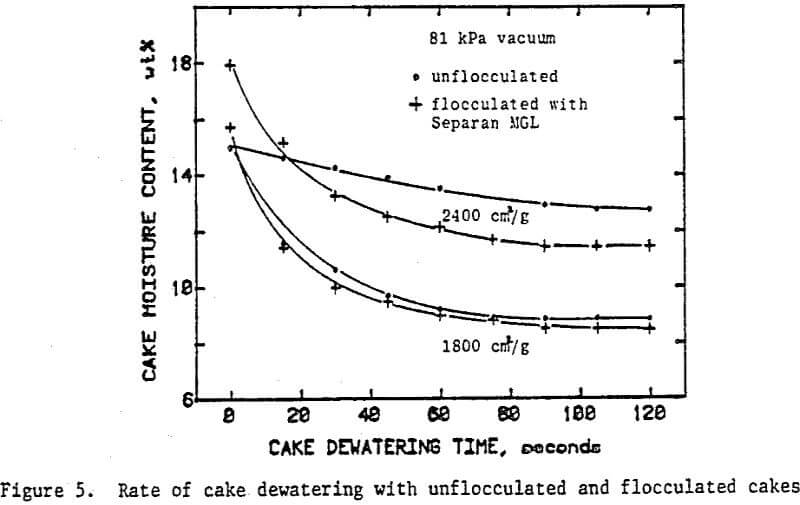
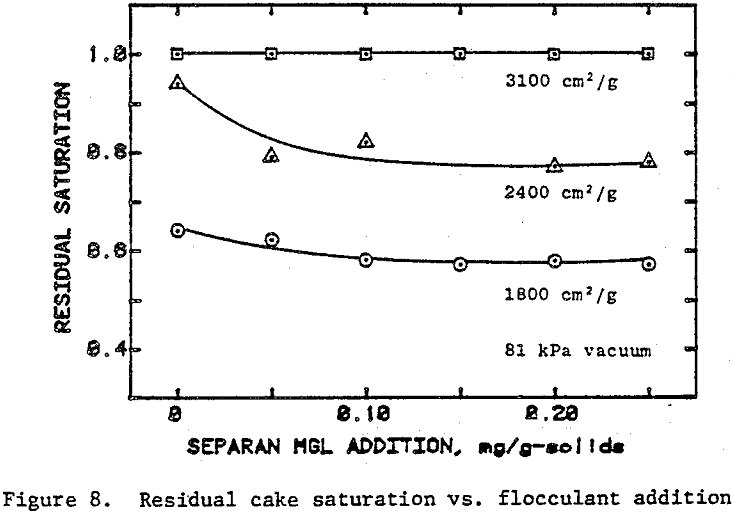
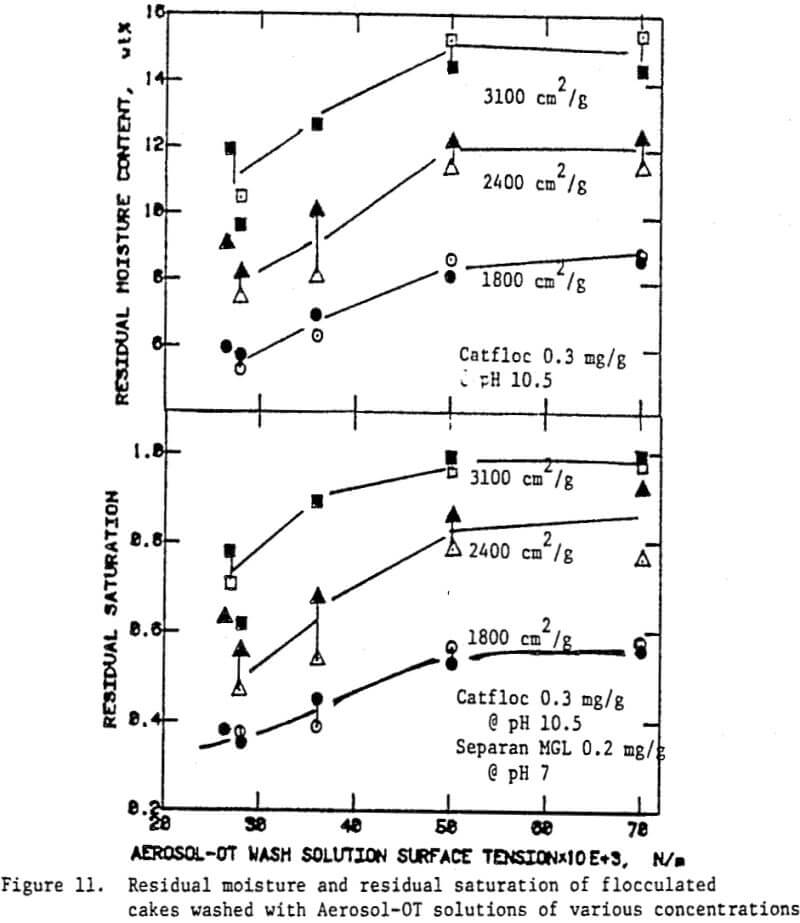
Once the minimum pressure required for the desaturation of filter cake was exceeded, cake dewatering took place in either the absence or the presence of airflow through the cake and filter medium until the equilibrium (residual) moisture was reached. Although the flocculated cakes were dewatered to slightly lower equilibrium moistures than the unflocculated cakes, the time taken to reach equilibrium was not affected.
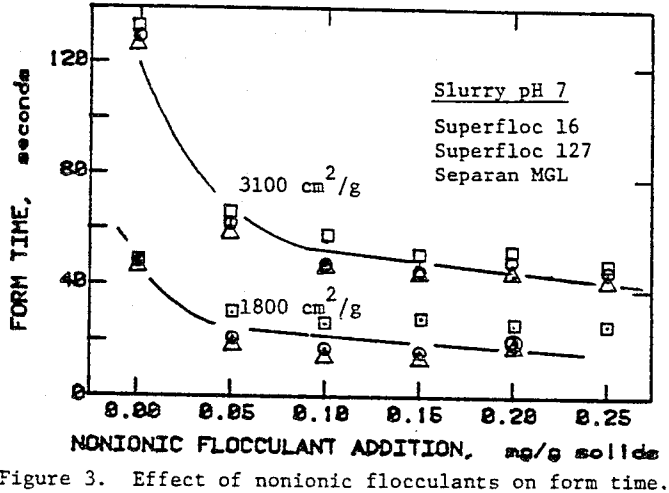
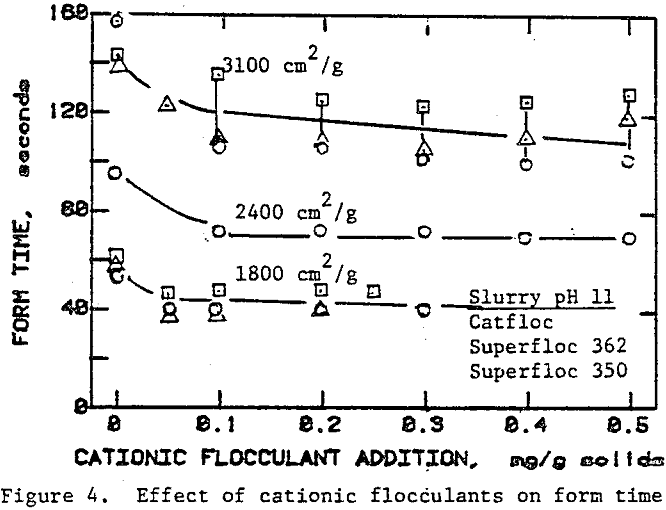
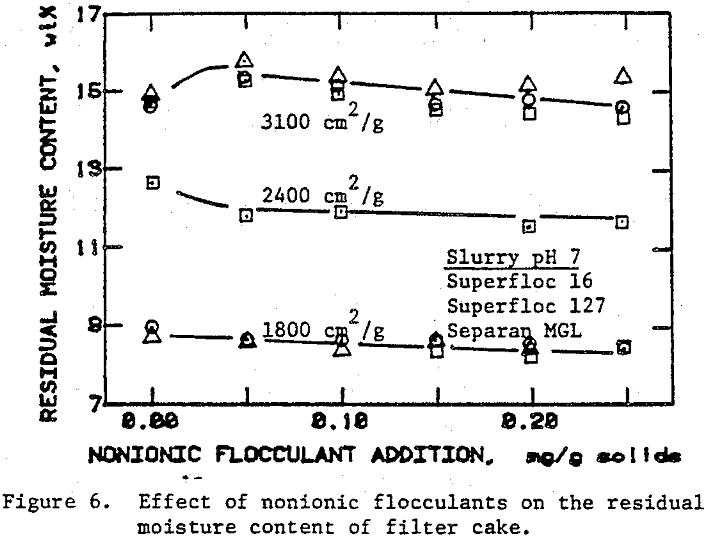
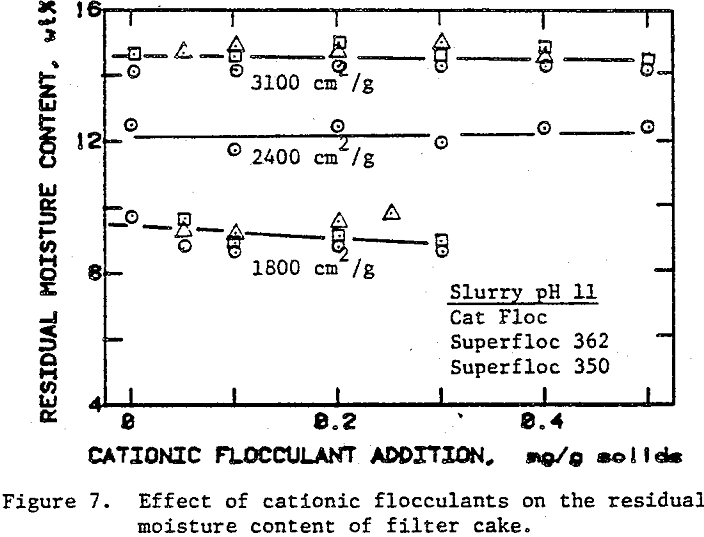
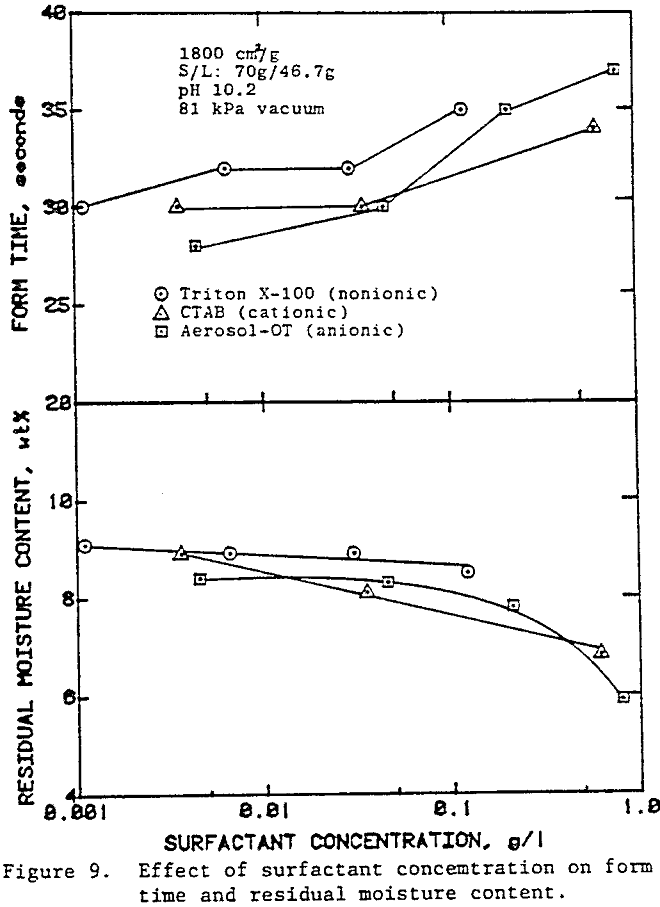
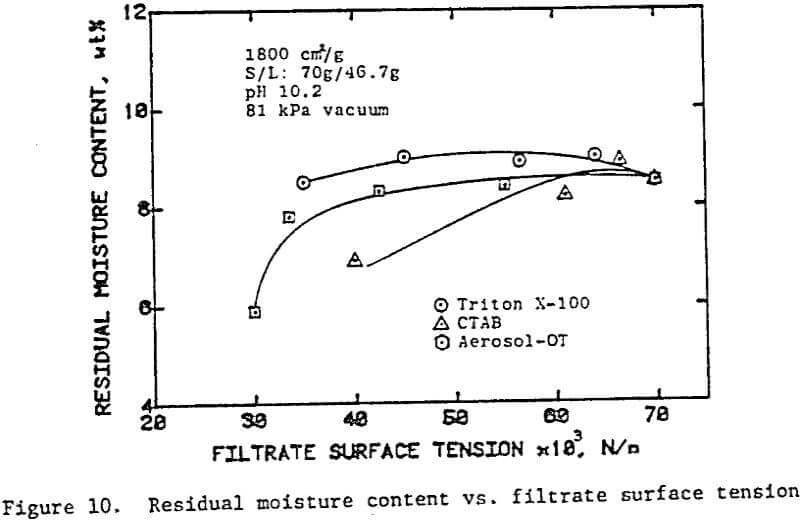
In the preceding sections we have seen that flocculants improve the cake formation rate, but they are not effective in reducing the residual moisture. On the other hand, surfactants have the potential for reducing the residual moisture. The use of surfactants with unflocculated slurries, however, caused severe dispersion of fine particulates which adversely affected cake formation.