Table of Contents
Biological leaching of low-grade ores has been gaining interest and acceptability in recent years. Sulfide ores, which are subject to oxidation of specific bacteria such as Thiobacilli, are being bioleached commercially. Other ore types, especially oxides and silicates, have been examined for their leachability by microorganisms. Ehrlich (1980), Holden and Madgwick (1983), Groudev (1987), and Bosecker (1989) have described some of these studies.
The Bureau of Mines (Perkins and Novielii, 1962) showed that manganese could be leached from several types of domestic ores by bacterial action. More recent studies on the bioleaching of manganese ores have focused on oxide ores, in particular ores in which the primary oxide MnO2 is reductively solubilized by the action of heterotrophic microorganisms. Manganese bioleaching studies have been reported by Madgwick (1987), Ehrlich (1987), Gupta and Ehrlich (1989), Agate and Deshpande (1977), and Groudev (1987). Hart and Madgwick (1986) reported that leaching of the Australian Groote Eylandt ore occurred by bioreduction under microaerobic (lower than atmospheric levels of free oxygen available) conditions when molasses was used as the nutrient source.
Microorganisms capable of solubilizing manganese bioreduction include Achromobacter spp., Bacillus spp., Enterobacter spp. and Aspergillus niger as reported by Ehrlich ( 1980) and Silverio and Madgwick (1985). They can be native to the ore or introduced as isolates cultured from soils, freshwater or marine environments, ore dumps or other ore deposits. Manganese reducing microorganisms have been found in aerobic, microaerobic, and anaerobic environments. Madgwick (1987) describes two mechanisms for the microbial reduction of manganese dioxide: direct, whereby the bacteria must come in contact with the mineral surface to enzymatically reduce the manganese dioxide, and indirect, in which the microorganisms produce reducing organic acids during fermentantive metabolism.
Most of the bioreduction studies reported in the literature, have been conducted in flasks or batch reactors. The objective of this research was to determine if bioreduction of manganese oxides occurs when low-cost heap leaching techniques are used.
Experimental
Ore
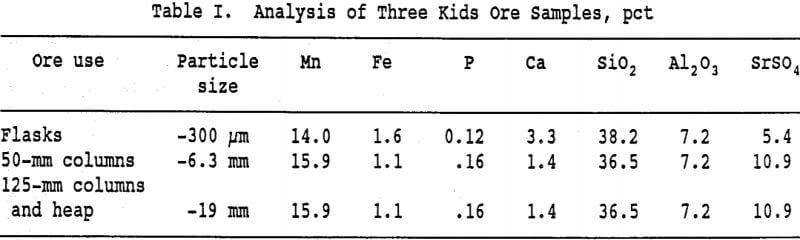
Manganese wad ore from the Three Kids deposit in Clark County, Nevada, was the principal ore used in this study. The deposit was described by Johnson and Trengrove (1956). Two samples of ore were obtained from the deposit. One sample was finely crushed for flask studies while the other sample was selectively crushed for column and heap leach experiments. The analyses of the principal constituents of these samples appears in Table I.
The Three Kids ore samples contained manganese oxides in a gangue of quartz, feldspar, gypsum, and clay. X-ray diffraction showed the ore to be primarily amorphous with pyrolusite (β-MnO2) as the only identifiable manganese oxide.
Flask leaching studies were conducted on three other manganese oxide ores to determine if the techniques developed in this study could be applied to similar ore types. The ore sources and their manganese concentrations are Salvador, Arizona (36.4 pct), Silver Cliff, Colorado (3.7 pct), and Algoma-Zeno, Minnesota (15.5 pct).
Media
Early flask and column studies were conducted using-a glucose-ammonium sulfate-potassium dihydrogen phosphate medium (4 g/L C, 2 g/L N, 1 g/L P). Later the medium was modified by eliminating the potassium phosphate. Molasses media were prepared from either a food-grade molasses or factory molasses, an inexpensive by-product of sugar cane processing. Both types of molasses contained 30 to 32 pct sucrose. Molasses cannot be filter-sterilized because of its high viscosity or sterilized by autoclaving without caramelizing. Consequently, media were prepared using as-received molasses and sterilized distilled water. The molasses was diluted to concentrations of 3-pct and 5-pct (w/v). No additional nutrients were added. To determine the amount of chemical leaching attributable to the media in the absence of microorganisms, 1 g/L sodium azide was added to the media in control tests. The mechanism by which azide inhibits oxidation and reduction was described by Brock (1979). Rosson et al. (1984) described the effectiveness of this microbiological inhibitor with manganese oxidizing bacteria, and Myers and Nealson (1988) reported on its use with a manganese reducer. Initial pH’s of the molasses media were 5.4 to 5.7 without sodium azide and 5.7 to 6.0 with the inhibitor.
Microorganisms
Inoculations with microorganisms were not required to initiate bioleaching, as organisms present in the ore and/or the molasses media were sufficient in some tests, inoculations were made using mixed cultures obtained from leach solutions which had yielded high extractions in a previous experiment with a similar medium.
A number of different bacteria were isolated from bioleach media, using spread plates of peptone-tryptone-glycerol-yeast extract agar. Bacterial identifications were made on the basis of the organisms morphological and cultural characteristics and Roche miniaturized multitest tubes for physiological characteristics.
Chemical Analysis
Culture fluids were analyzed for soluble manganese and other metals by Inductively Coupled Plasma (ICP) spectroscopy. Ore head and tail samples were also analyzed by ICP. Total carbon in the culture fluids was determined by a high temperature combustion analyzer with infrared detection.
Shake-Flask Tests
The shake-flask experiments were conducted with 2 g of minus 300-µm ore and 100 mL of medium in 250 mL stoppered Erlenmeyer flasks (2 pct pulp density). Each stopper contained a septum-covered sampling port and a vent port to which a 0.22-µm air filter was attached. The flasks were continuously shaken on an orbital shaker at 200 rpm in a temperature controlled room (22° C ± 3° C). In some tests the ore was sterilized by autoclaving in the flasks at 121° C prior to addition of the medium. Other tests used unsterilized ore, but the flasks were autoclaved before use to prevent the introduction of contaminants. Sampling was performed aseptically. All flask tests were conducted in triplicate and the data were averaged.
Column Tests
No attempt was made to sterilize the ore, media or equipment used in the column tests. The columns were capped with lids containing a small air hole. This limited the amount of air in the column in an effort to establish microaerobic conditions in the ore bed. Two column sizes were used: 50-mm I.D. and 125-mm I.D. The smaller columns contained 500 g of minus 6.3-mm ore and were leached using 5 L of medium, while the larger columns contained 9 kg of minus 19-mm ore, and 20 L of medium were used. For each column, the medium was pumped from a reservoir onto the top of the ore bed and the column effluent was pumped back into the reservoir so that the medium was continuously recycled. The media were completely replaced with freshly prepared media every 4 to 6 weeks to ensure an ample nutrient supply and to eliminate toxic metabolite build-up. Samples of media were collected and analyzed weekly and the data for each set of replicates were averaged. The columns were run under the same temperature conditions as the flasks, 22 ± 3° C.
Heap Test
The open design of a heap allows airborne microbes to be introduced onto the ore. This parameter was studied by bioleaching 35 kg of minus 19-mm ore in a 33-cm I.D. cylindrical container. The ore bed was 40 cm high and the container was open to the atmosphere. Twenty liters of 3-pct food-grade molasses medium were dripped onto the heap at 0.03 L/min/m through a system of emitters laid out on the surface of the ore. After the weekly samplings, fresh medium was added to the reservoirs to compensate for evaporation and consumption of nutrients by the microbes. The medium was replaced when the manganese concentrations and the pH measurements indicated that leaching was no longer occurring. The heap was leached at ambient temperature with no temperature controls.
Results and Discussion
Preliminary Studies
Shake-flask screening tests were conducted to provide information for selecting media and operating conditions for the column and heap bioleach experiments. A variety of media were evaluated as nutrient sources for heterotrophic microorganisms to biologically leach the manganese from the Three Kids ore. Promising results were obtained with a glucose-ammonium sulfate-potassium phosphate medium. Indigenous microbes were cultured, and bacteria and fungi were isolated from the mixed cultures. Inoculations of isolates into slurries of sterilized ore and media showed that the bacteria solubilized more manganese and used less carbon than did the fungi. Subsequent studies with the glucose-mineral salts medium showed that the presence of phosphate favored the growth of fungi, which caused depletion of carbon from the medium and an increase in medium pH. When potassium phosphate was eliminated from the medium, a mixed bacterial culture extracted up to 47 pct of the manganese from the ore in flask tests in 6 weeks.
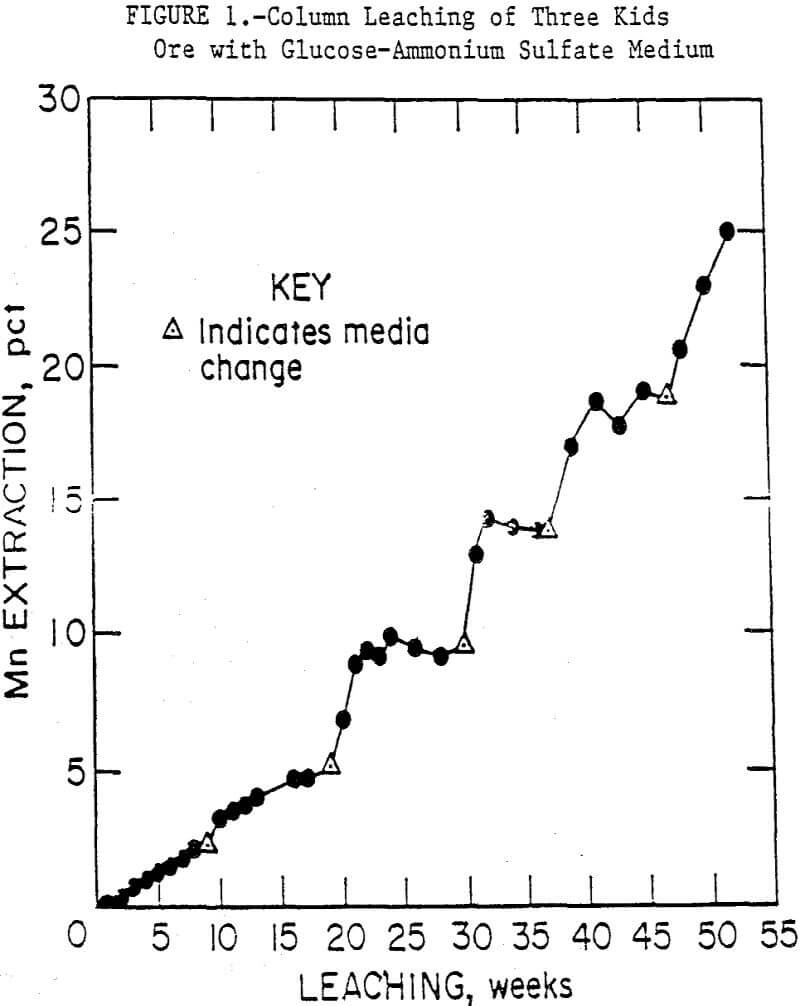
Column leach experiments were conducted using the minus 6.3-mm ore with the glucose media in the 50-mm columns. As with the shake-flask tests, medium which contained potassium phosphate favored the production of fungi and, consequently, rapid carbon depletion with a corresponding rise in pH. Also, manganese that had initially been solubilized precipitated from solution as Mn3(PO4)2·3H2O, a compound insoluble in all the common aqueous and organic solvents. Much of the precipitate adhered to the ore. When a medium consisting only of glucose and ammonium sulfate was used, manganese was solubilized as long as glucose was available, and little precipitation occurred. The manganese concentrations in the medium would stabilize when the glucose was metabolized, and the pH would rise, although not as high as in the phosphate medium. When the medium was replaced or solid glucose was added, leaching resumed. Column bioleaching of minus 6.3-mm Three Kids ore, with periodic replacement of the glucose-ammonium sulfate medium, extracted up to 28 pct of the manganese in 52 weeks. These results are summarized in figure 1.
The cost of glucose, and other refined sugars, make their large-scale application as bioleaching nutrients prohibitively expensive. A cheaper source of organic carbon is necessary for bioheap-leaching to be economic. When the shake-flask screening tests showed that using less expensive molasses to feed the microorganisms gave better results, subsequent studies were undertaken with media prepared by diluting molasses with sterile water.
Shake-Flask Tests
Shake-flask tests were conducted with food-grade molasses media, and a number of experimental variables were evaluated. The data are too numerous for presentation here, but several conclusions can be drawn from these experiments:
- Similar extractions were obtained from the sterilized ore and from the nonsterilized ore. This suggests that microorganisms were introduced by the non-sterile molasses.
- Media inoculated with mixed populations of microorganisms cultured from previous experiments extracted more manganese than the uninoculated media did.
- Media which contained the microbial inhibitor sodium azide solubilized significantly less manganese than did biologically active media.
- Manganese extraction was a function of the carbon content of the medium.
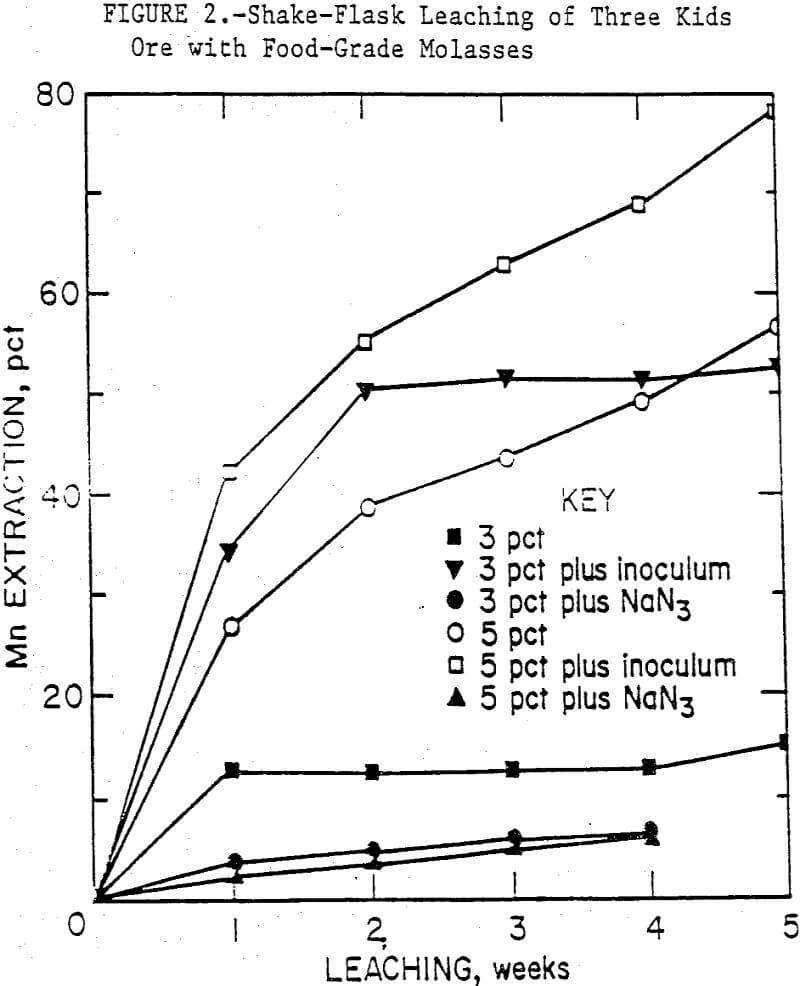
Some typical results from leaching Three Kids ore with food-grade molasses are shown in figure 2. After 5 weeks of leaching, 78 pct of the manganese was solubilized from the ore with inoculated 5-pct medium compared to 53 pct manganese extraction with inoculated 3-pct medium.
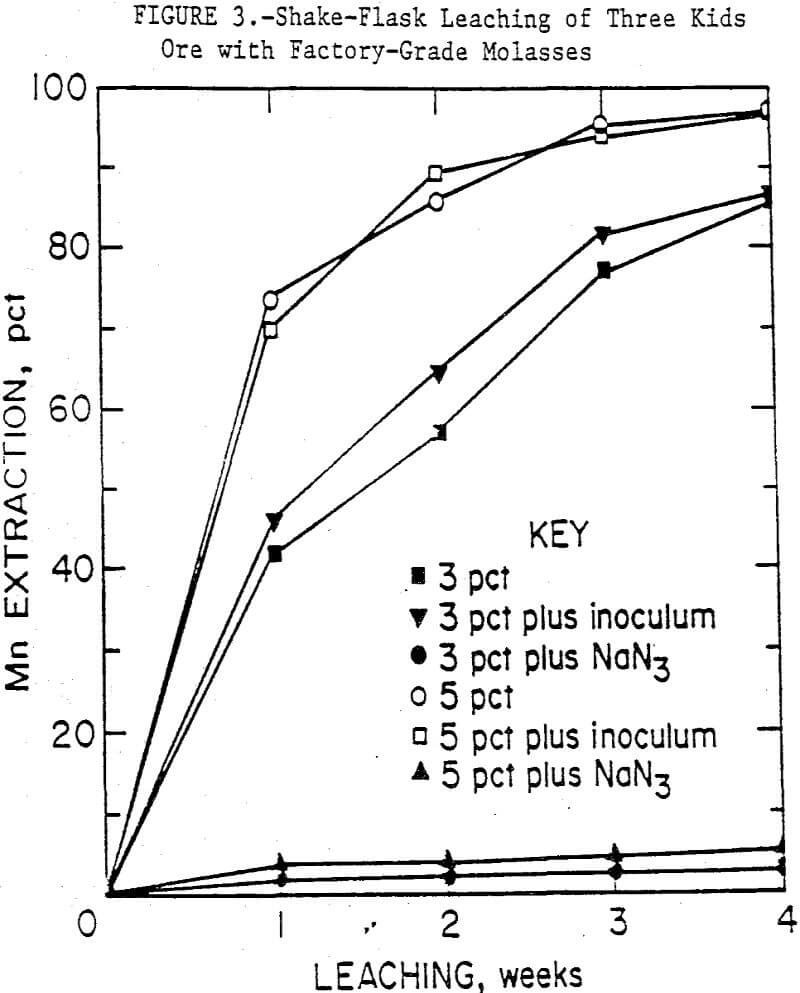
Figure 3 shows the results from leaching Three Kids ore with factory molasses. The 5-pct medium solubilized 97 pct of the manganese in 4 weeks while the 3-pct medium extracted 87 pct, both considerably higher than observed with food-grade molasses. In this case, little difference was observed in manganese extraction between inoculated and uninoculated tests. The additional processing of the raw sugar that is required to produce food-grade molasses could inhibit microbial action of some species of bacteria. Studies are in progress to determine the origin of the microorganisms responsible for the bioreduction of manganese, i.e., whether they come from the ore, the medium, or both.
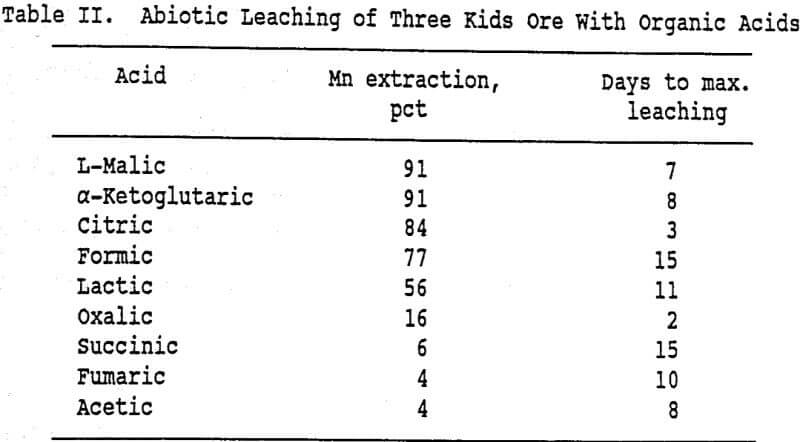
A series of abiotic shake-flask leaching experiments were conducted to evaluate whether organic acids produced in the metabolism of glucose by respiration and fermentation pathways are capable of solubilizing manganese from Three Kids ore. The tests were conducted under the same conditions as the bioleach experiments, and the carbon concentration of each leach solution was 4 g/L. The metabolites examined were acetic, citric, formic, fumaric, lactic, α-ketoglutaric, L-malic, oxalic and succinic acids. The results are shown in Table II.
Although L-malic and α-ketoglutaric acids extracted the most manganese (91 pct each), citric acid leached the fastest (84 pct in 3 days). Sodium azide was added to some of the leach solutions (1 g/L) to determine if it chemically inhibited the acids. The amount of manganese extracted in these flasks was the same as in the flasks without sodium azide. These experiments suggest that an indirect leaching mechanism, i.e., leaching by organic acid metabolites, contributes to the solubilization of manganese from the ore. Examination of solution oxidation potential and pH data indicate that leaching is not the result of acid generation, but probably involves reduction of the higher oxides of manganese to soluble manganous ions by the organic compounds. However, these findings do not rule out the participation of a direct
bacterial leaching mechanism. It is likely that both direct and indirect leaching is occurring in these bioleach experiments, as Madgwick (1987) suggested from his bioleach studies.
Column Tests
Three sets of column experiments were conducted using molasses media. In the first set, four 50-mm columns of the ore were leached using the 3-pct food-grade molasses medium. Two of the columns were initially inoculated with 25 mL of a mixed culture grown from a flask test which used the same medium. The other two columns were not inoculated. In a second four-column set, 5-pct food-grade molasses medium was used. Sodium azide was added to the medium for one of the columns. The 125-mm columns and the 3-pct medium were used for the third set of two columns. The media were dripped onto the ore in the 50-mm columns at 0.7 L/min/m² while on the 125-mm columns the rate was 0.3 L/min/m².
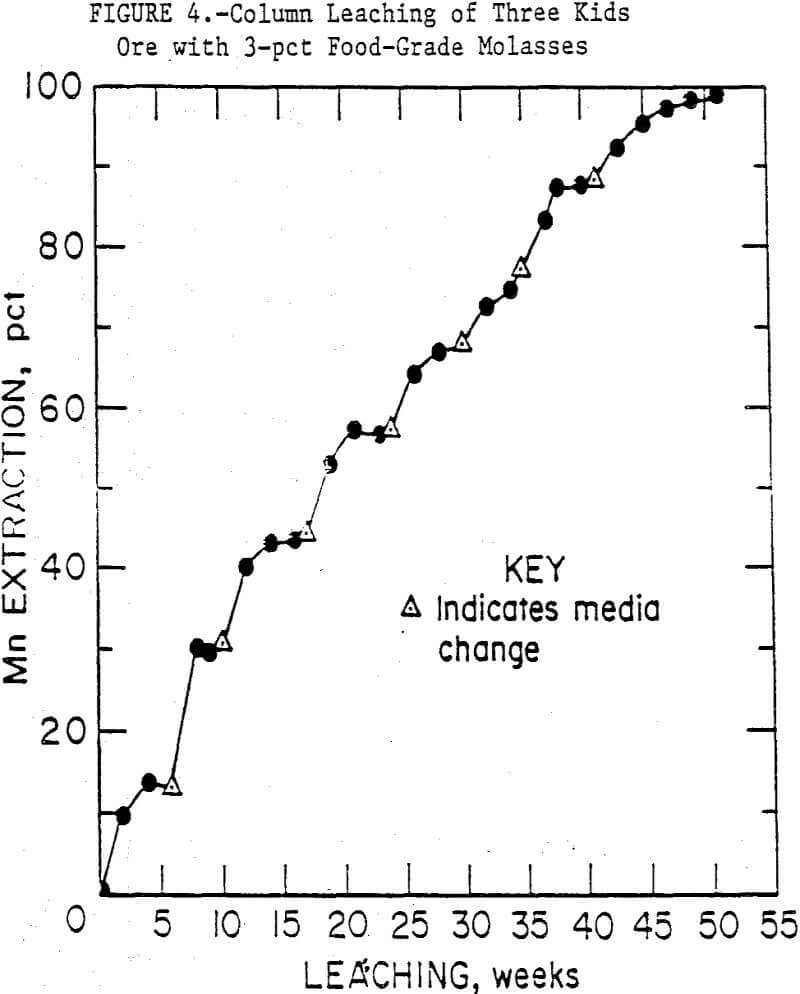
The course of manganese solubilization in the first set of 50-mm columns, using 3-pct food-grade molasses, is shown in figure 4. Initially, the inoculated columns showed a higher rate of manganese solubilization, but by the second week this difference was no longer evident. Therefore, the points in figure 4 are averages of the inoculated and uninoculated column data. After 50 weeks of continuous leaching, and six media changes, 99 pct of the manganese in the columns had been solubilized. The need for media changes was signalled by an increase in pH from 5.5 to > 6.5 and a stabilizing of manganese concentrations in the media. As bioleaching continued, the ore particles disintegrated and became covered with brown microbial slime. Microscopic examination of this slime showed it contained bacteria, fungi and possibly algae. Fungi were obvious in the media reservoirs, but not as apparent in the columns.
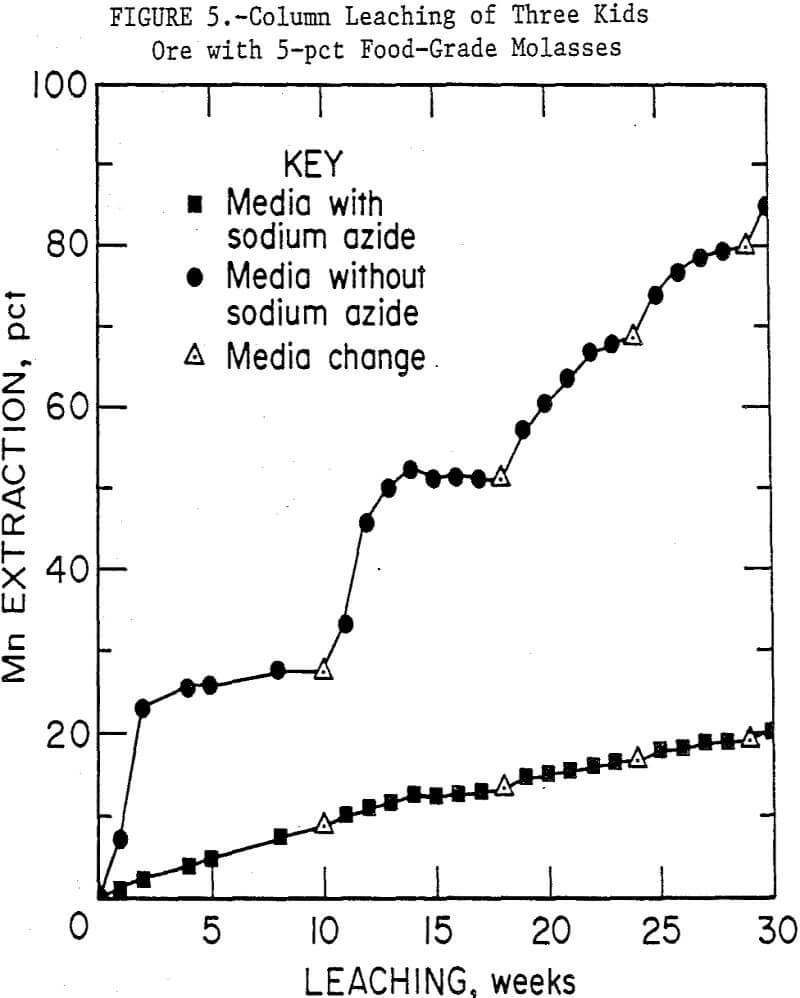
Figure 5 compares the results of using 5-pct food-grade molasses medium with and without the sodium azide microbial inhibitor. In 29 weeks, the biologically active media leached an average of 80 pct of the manganese from the ore, while the azide containing medium leached only 19 pct. The ore in the columns without sodium azide took on the appearance of the ore in the previous set, i.e., brown biomass was very evident. But the ore in the column with sodium azide retained its original black color and structure, and no growth of biomass was observed. One bacterium was isolated from the medium in the column, a gram-negative, facultative-anaerobic rod. It has not yet been identified. Although sodium azide is a metabolic inhibitor, this bacterium may be resistant to its effects.
The 125-mm column experiments are still in progress. Approximately 11 pct of the manganese has been extracted after 29 weeks of leaching. The minus 19-mm ore particles are leaching at a substantially slower rate than the minus 6.3-mm particles used in the 50-mm columns. Channeling is apparent in the ore column and may be inhibiting the leach rate. Fungi growth and slime on the ore are more evident than in the smaller columns.
Heap Test
The lab-scale heap test has extracted 4 pct of the manganese from the ore in 39 weeks. During this period, the 20 L of medium was replaced nine times. As in the column experiments, the increase in medium pH and the stabilizing of manganese concentrations in the medium were used to indicate the need for fresh medium. Initially the medium was replaced every six to eight weeks. However, the medium is now replaced every two to three weeks in order to provide an adequate supply of nutrients for the microorganisms. Fungi are much in evidence.
The same ore size (minus 19-mm) has been used in the 125-mm columns as in the heap. However, the media delivery system for the heaps delivers only 0.03 L/min/m² compared to the drip rate of 0.3 L/min/m² in the 125-mm columns.
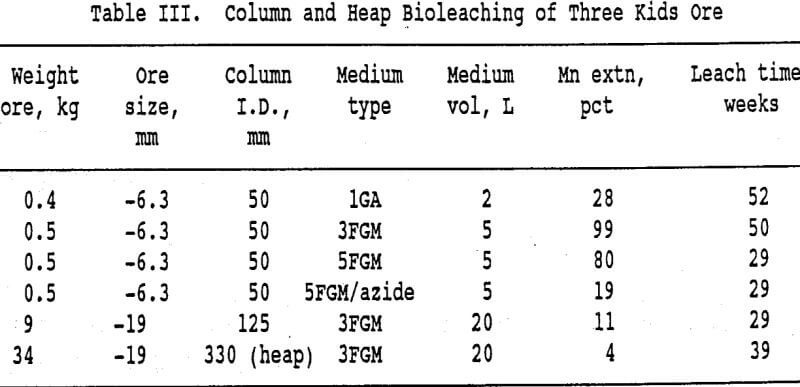
This has resulted in less manganese extraction from the heap than from the columns in a comparable time period. The higher media to ore ratio in the columns is also a factor. Work on improving the heap and delivery system designs is in progress. The results from the column and the heap experiments are summarized in Table III.
Identification of Microorganisms
A survey of the microorganisms present in various bioleach solutions is being conducted. Molasses supports the growth of a wide variety of microorganisms. Isolates identified to date include the common soil bacteria Enterobacter agglomerans, E. cloacae. Pseudomonas cepacia, and Bacillus coaqulans. Klebsiella oxytoca and Deinococcus radiodurans have also been found in some of the solutions. Madgwick (1987) reported that of the four microbial isolates responsible for the reductive solubilization of manganese from Australian manganese dioxide tailings (E. agglomerans. E. cloacae. Aspergillus niger, and an Achromobacter sp.) the Enterobacter spp. were consistently selected out under microaerobic conditions. In the microaerobic conditions of columns and heaps, facultative anaerobic bacteria, such as E. agglomerans should easily survive.
Shake-Flask Tests with Other Oxide Ores
Shake-flask bioleaching experiments using Algoma-Zeno, Silver Cliff, and Salvador ores were conducted in a manner similar to the Three Kids tests. Each flask contained 2 g of minus 300-pm ore and 100 mL of 5-pct food-grade molasses medium. None of these flasks were inoculated. In 4 weeks of leaching, 97 pct of the manganese was solubilized from the Algoma-Zeno ore and 98 pct was solubilized from the Silver Cliff ore. The higher grade Salvador ore (36.4 pct manganese) required two aliquots of media (100 mL each) to solubilize 99 pct of the manganese in six weeks.
Summary
Three Kids low-grade manganese wad ore was bioleached with molasses media in flask, column and lab-scale heap experiments. More than 97 pct of the manganese was bioleached from minus 300-µm ore in shake-flask tests conducted for 4 weeks with a 5-pct factory molasses medium and a 2 pct pulp density. Column tests using 3—pct food-grade molasses medium leached 99 pct of the manganese from minus 6.3-mm ore in 50 weeks and 11 pct of the manganese from minus 19-mm ore in 29 weeks. Small heap bioleaching of the minus 19-mm ore with 3-pct food-grade molasses removed about 4 pct of the manganese in 39 weeks. Preliminary tests showed that other manganese oxide ores were also bioleachable with molasses as the sole nutrient source. Studies to optimize nutrient composition, liquid-to-solid ratios, ore particle size and heap design are in progress.