Table of Contents
The origins of AEROPHINE 3418A promoter date back to the 1960’s, when it was established that sulfide mineral collectors based on phosphine chemistry demonstrated efficacy. This new collector chemistry was found to provide flotation performance differing significantly from those of more traditional thiol collector chemistries such as xanthates, dithiophosphates, mercaptobenzothiazoles, thionocarbamates and those with xanthogen groups.
Chemistry And Handling Properties
AEROPHINE 3418A is an aqueous solution of sodium diisobutyldithiophosphinate. The structure of this active ingredient is as follows:

It is manufactured by first reacting phosphine (PH3) with isobutylene (C4H8) to produce diisobutylphosphine [(C4H9)2PH]. This intermediate is then reacted with sulfur and caustic to produce the final product, AEROPHINE 3418A promoter. As a result of some by-product formation in the manufacturing process, the final product contains a low level of triisobutylphosphine sulfide [TIBPS-(C4H9)3PS].
If any crystallization occurs, the product must be warmed, preferably to 15°C or above, and agitated in order to redissolve the crystals of active ingredient and TIBPS.
Application
AEROPHINE 3418A has found widest application in flotation of copper- and lead-sulfide minerals, particularly where these are found in complex sulfide ores containing sphalerite zinc mineralization, and ores with high levels of pyrite and/or pyrrhotite.
Laboratory testing in the zinc flotation stage with some ores has given indications that 3418A may be effective as an activated sphalerite collector. The results when using AEROPHINE 3418A promoter as an activated sphalerite collector, generally have not as frequently shown the advantages more typically seen of its application to copper, lead and precious metals flotation stages.
There are two basic tenets to follow for proper evaluation of the potential metallurgical benefits AEROPHINE 3418A may provide: 1) use staged additions of 3418A for optimum selectivity and control, and 2) use 3418A as the sole collector. Adherence to these two tenets is strongly recommended.
If a visual assessment of this first flotation stage is favorable, a second addition of 3418A should be made at about 25% of the currently used collector dosage. The concentrate from this second flotation stage should be kept separate from the first. If a visual assessment seems to indicate more collector is required, a third flotation stage should be carried out in a like manner to the second stage, keeping this concentrate separate from the others.
AEROPHINE 3418A generally displays little or no frothing properties. At times this has required an increase in frother dosage when using 3418A as collector, compared to the frother dosage required with a collector having frothing properties. This has proven advantageous in some plants, as it tends to separate the function of collector from that of frother.
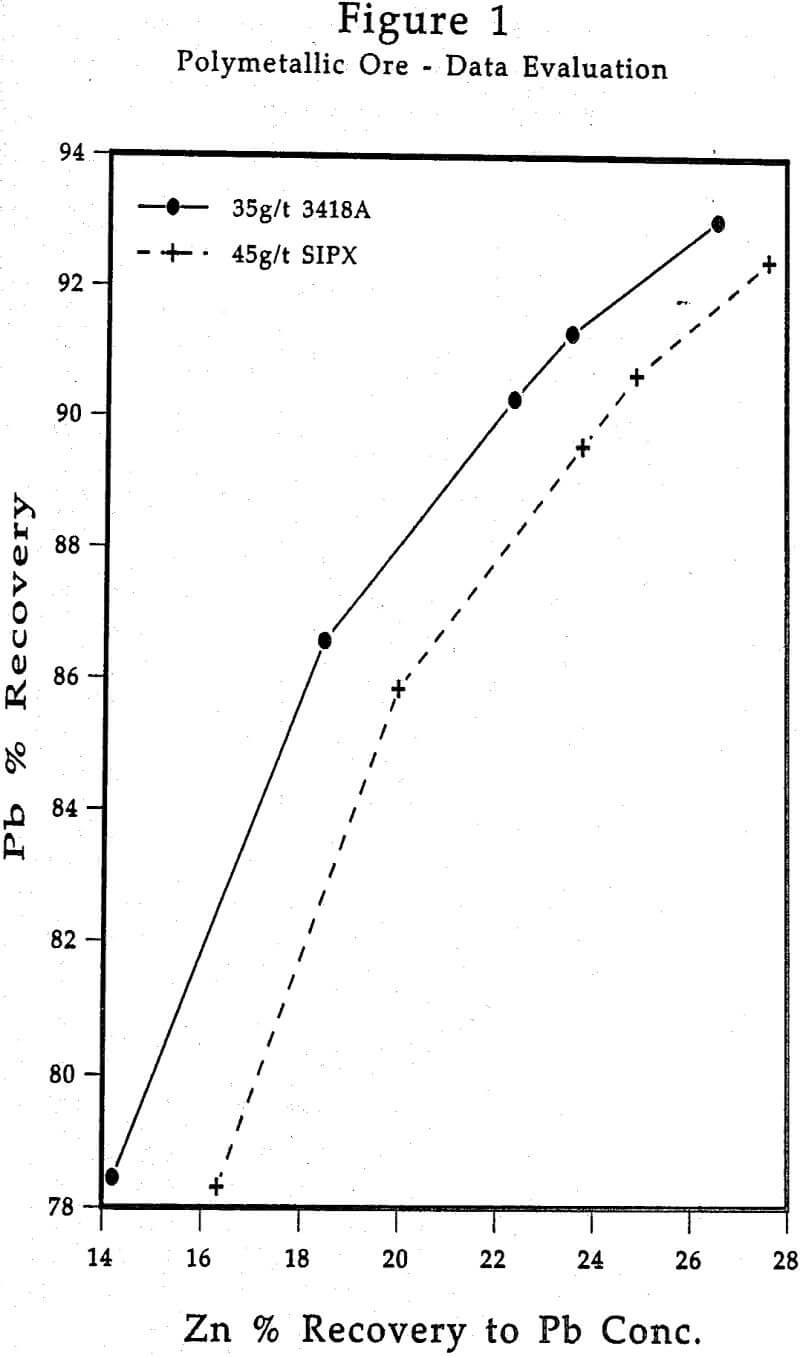
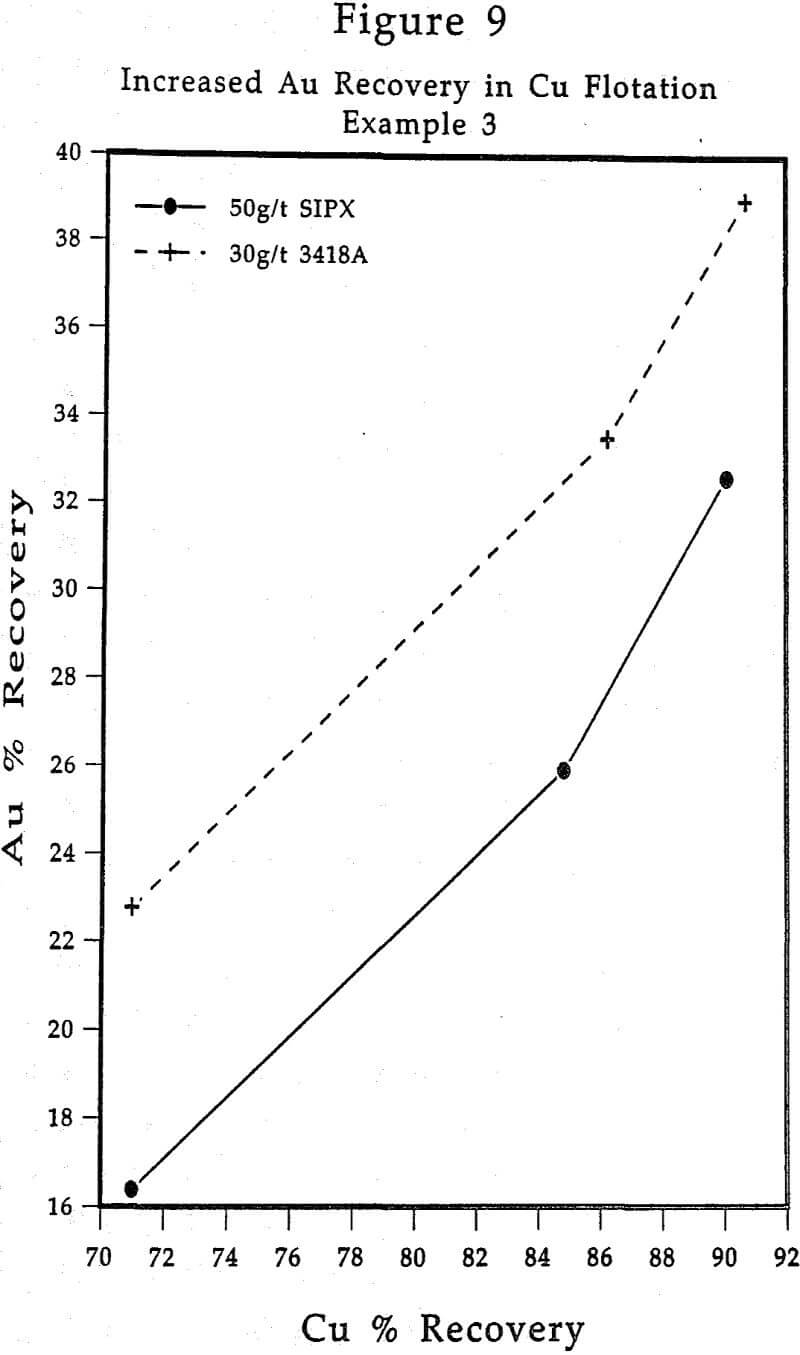
Having assay results in hand from the flotation experiments conducted, the metallurgical data will require evaluation. Particularly when dealing with Cu-Zn or Pb-Zn ores, very frequently containing Au and/or Ag values, certain evaluation techniques tend to best establish and highlight the performance characteristics of AEROPHINE 3418A.
In this instance, it would be very informative to plot Ag recovery to the Pb concentrate vs. Pb recovery, and Zn recovery to the Pb concentrate vs. Pb recovery. This will help provide a clear picture of where Ag and Zn are reporting in relation to some comparative unit recovery of the primary metal being recovered, viz., lead.
Every “rule” has its exceptions, however, and there are a number of plants presently treating Cu-Zn ores, using AEROPHINE 3418A promoter and a dithiophosphate in the Cu flotation circuit. Generally there are significant precious metals values and secondary copper mineralization associated with chalcopyrite at these plants. In these circumstances the conjunctive use of AEROPHINE 3418A promoter with a dithiophosphate as a secondary collector has been shown to be an advantageous collector combination, without negative impact on the resulting metallurgy. Other thiol type collectors may demonstrate suitability as secondary collectors with 3418A, as well.
The conjunctive use of xanthates with AEROPHINE 3418A promoter, for Cu or Pb flotation, is very strongly discouraged. There are overwhelming data to show that such joint reagent usage diminishes the benefits provided by the 3418A.
When the use of SIPX was eliminated, and AEROPHINE 3418A promoter was used as the sole collector at 43% of the normal SIPX dosage rate, a significant improvement in metallurgy was noted. AEROPHINE 3418A has since been adopted as the collector at this plant, used without any other collector type. The dosage of 3418A has ultimately been optimized at 10% of the former xanthate consumption.

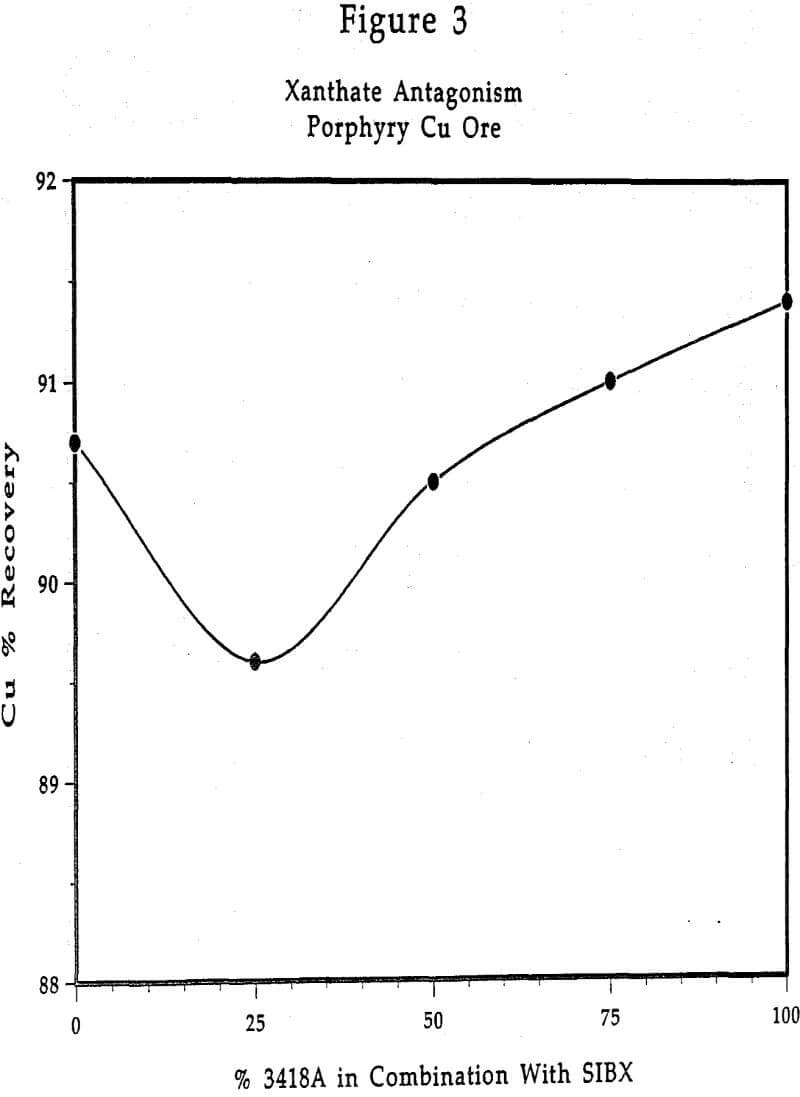
In order to study the antagonistic effect xanthate has when used with AEROPHINE 3418A promoter, laboratory flotation tests were made on a porphyry copper ore. Sodium isobutyl xanthate (SIBX) and 3418A were evaluated in different proportions from 100% SIBX to 100% 3418A, at a constant total collector dosage.
The only area of application where the use of xanthate with AEROPHINE 3418A can be recommended is for activated sphalerite flotation. AEROPHINE 3418A generally has not demonstrated advantages in use as the sole collector for sphalerite as frequently as it has for the flotation of copper minerals and galena. As mentioned previously, this is thought to be due to the stoichiometry of Fe within the sphalerite crystal lattice. Use of a xanthate with 3418A in Zn flotation has given promising results with some ores.
Advantages in Use
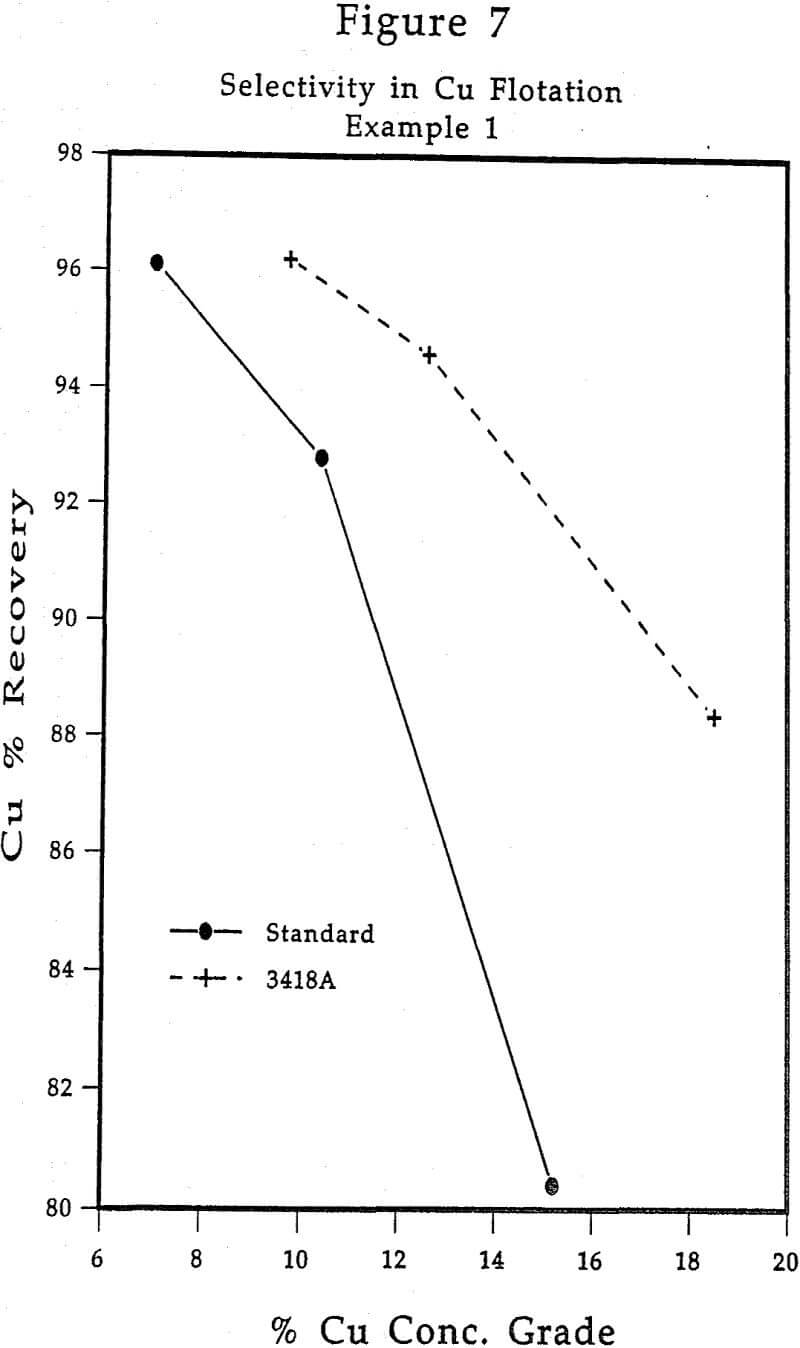
A laboratory copper flotation investigation was carried out on a Cu-Zn ore containing 0.74% Cu and 2.92% Zn lime, zinc sulfate and sodium cyanide were used in the grinding stage. The standard collector combination for this ore was 10 g/t xanthate plus 10 g/t dithiophosphate, with a copper circuit pH 9. The use of 3418A produced significantly improved Cu concentrate grades vs. recoveries, compared to use of the standard collectors, with the same overall recovery. The improved concentrate grades were due to increased selectivities against both sphalerite and pyrite.