Table of Contents
Metal separations and metal recoveries by ion exchange and solvent extraction of their thiocyanate complexes are widely practiced in analytical chemistry as well as in some industries. Consequently, a critical review of the chemistry of thiocyanic acid and thiocyanates is presented in the second part with the aim of providing a clearer understanding on the causes of these problems and giving options as how to minimize them.
Metal Thiocyanate Systems
Similar to hydrochloric acid, thiocyanic acid (a psudohalogenic acid) forms stable thiocyanato complexes with a large number of metals.
The liquid ion exchange reactions involved are given below.
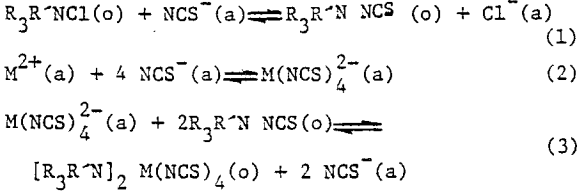
The extraction efficiency follows the order Zn>Co>Cu>Fe>Mn>Ni.
Zn²+(a) + 2 SCN- (a) ↔ Zn(NCS)2(o)……………………………………………………………..(4)
This equation applies over the whole pH range for Zn(II) in the absence of any complexing anions. The extraction is quantitative and can be used in the preparation of pure zinc thiocyanate and in the separation of zinc and cadmium.
Thiocyanic Acid and Thiocyanates
Gaseous thiocyanic acid is readily soluble in water, alcohol and benzene. On cooling the gas to -30° to -40°C a white crystalline solid HNCS results, but it is difficult to obtain the pure white solid on account of condensation of moisture which causes the formation of yellow condensate.
HNCS + H3O+ + H2O → H2S + CO2 + NH4+……………………………………………..(5)
HNCS + H2S → CS2 + NH3………………………………………………………………………..(6)
KNCS + 2 H2SO4 + H2O → COS + KHSO4 + NH4HSO4……………………………………(7)
COS + H2O → H2S + CO2………………………………………………………………(8)
COS + C2H5OH → C2H5SH + H2O……………………………………………(9)
NH4NCS → HN4CN + S(sol)………………………………………………………..(10)
The insoluble sulfur forms aggregates of sub-microscopic particles which are responsible for the pink color. These submicrons can slowly unite to form particles of microscopic size which are colorless (the spontaneous fading of the color). This process of coagulation is accelerated by the addition of potassium cyanide or by UV light of shorter wavelength (passed by quartz). The actual extent of decomposition is very slight, and in dilute solutions negligible.
2 HNCS + ½ O2 → (SCN)2 + H2O……………………………………………..(11)
3(SCN)2 + 4 H2O → 5 HNCS + HCN + H2SO4…………………………………….(12)
The intermediate product, carbonyl sulfide, formed via the hydrolysis of thiocyanic acid is highly flammable, burning with a slightly luminous blue flame.
2COS + 3O2 → 2SO2 + 2CO2………………………………………………………….(13)
The reaction between hydrogen peroxide and thiocyanate takes place according to different mechanisms, depending on the acidity of the solution.
4H2O2 + SCN- → HOCN + HSO4- + 3H2O………………………………………………(14)
HOCN + 2H2O → NH4+ + HCO3-…………………………………………………………..(15)
As far as the acid-catalyzed reactions are concerned, the main products are sulfate and hydrogen cyanide, but sulfur dicyanide S(CN)2, is also formed.
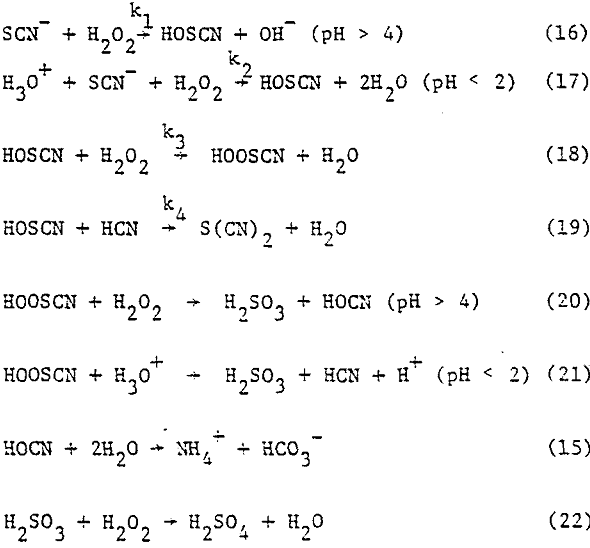
HNCS + 2HNO3 → HCN + H2SO4 + 2NO…………………………………………….(23)
The oxidation of cyanide often yields cyanate which in acid media decomposes via:
HOCN + H2O → NH3 + CO2…………………………………………………….(24)
Ferric Salts. It has been noted by some investigators that the gradual diminution in color of ferric thiocyanate solutions is associated with a reduction of Fe(III) to Fe(II). The study of mixtures containing a constant quantity of ferric salt and varying quantities of thiocyanate has shown that, while at the start the reduction is slowest in those solutions with the least thiocyanate, it proceeds at a more even rate, until ultimately the extent of reduction is greatest in these cases.

6Fe³+ + SCN- + 4H2O → 6Fe²+ + HCN + H2SO4 + 5H+……………………………………………..(32)
3 HNCS → H2N2C2S3 ↓ + HCN…………………………………………………………………………(33)
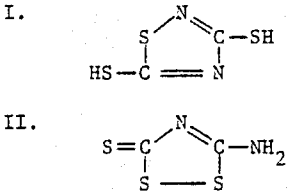
In the years that followed, this compound was known variously as perthiocyanic acid, iso-perthiocyanic acid and xanthanwasserstoff. The close relationship of these acids was demonstrated by Klason, who showed their interconvertibility by the action of acid or base.

After a great deal of speculation, the structure of isoperthiocyanic acid was established from x-ray crystallography and infrared spectrophotometry as II.

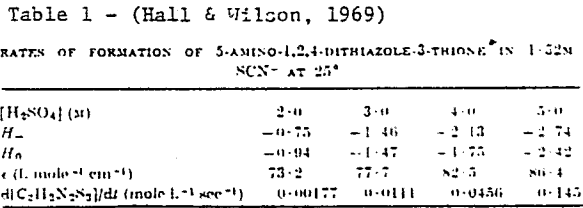
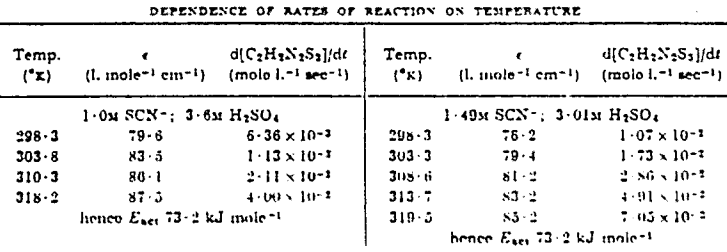
To explain the observation of third-order dependence on thiocyanate concentration and first-order dependence on Hammett acidity function, the following mechanism is suggested.
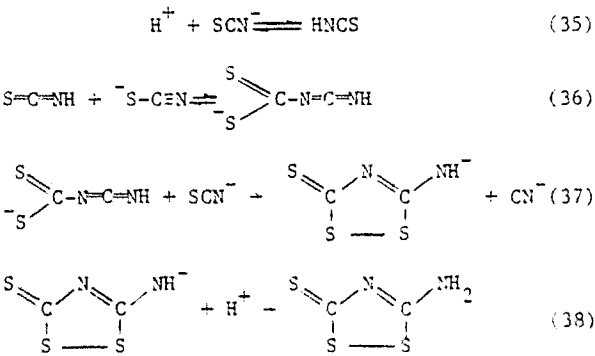
Chemical Reactions. Isoperthiocyanic acid is easily hydrolyzed either by heating with water at 200°C under pressure or by heating with strong sulfuric acid. Thiourea, carbonyl- sulfide and sulfur are formed.
H2N2C2S3 + H2O → H2NCSNH2 + COS + S………………………………..(39)
H2NCSNH2 → NH4NCS……………………………………………………………(40)
COS + H2O → CO2 + H2S………………………………………………………..(8)
H2N2C2S3 + 2H2O → NH4NCS + CO2 + H2S + S……………………………..(41)
H2N2C2S3 + 14[o] + 2H2O → 2CO2 + 3H2SO4 + N2…………………………..(42)
Nitric acid readily and completely decomposes isoperthiocyanic acid. When the finely powdered substance is added to the strong acid, N2, CO2 and NOx are evolved, and sulfuric acid and a little’ammonium salt are found in the liquid.
H2N2C2S3 + H2 → H2NCSNH2 + CS2………………………………………..(43)
Discussions
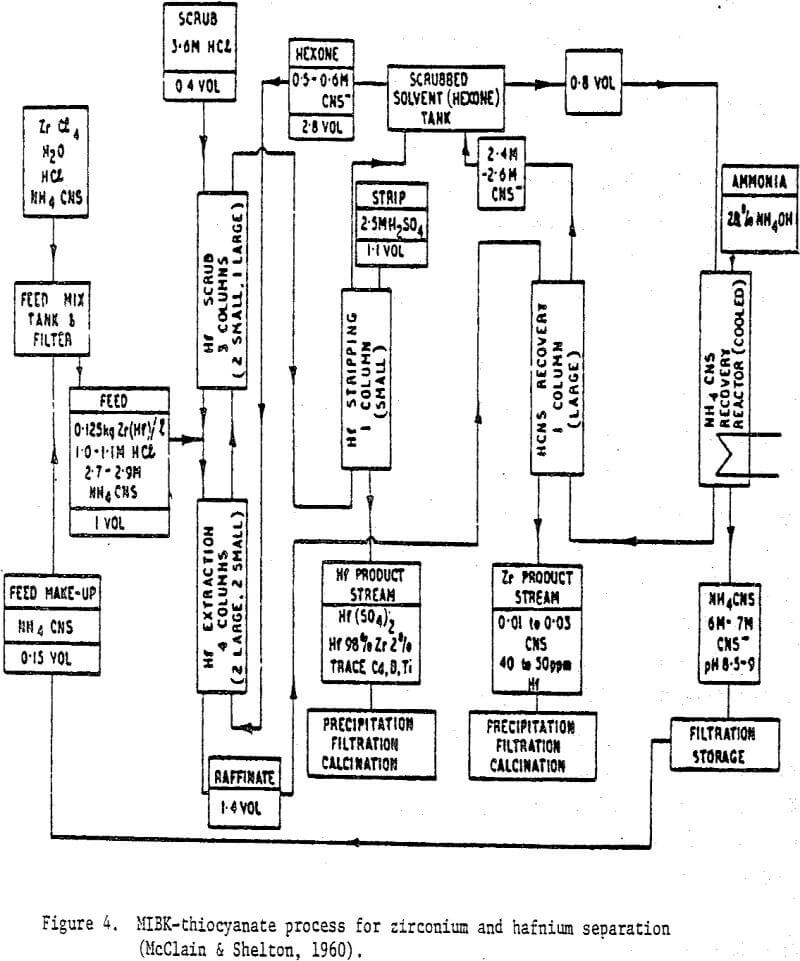
The metal thiocyanate systems presented in this review which are worth noting include the separation of scandium from rare earths and gallium from aluminum by ion exchange, and by solvent extraction of hafnium from zirconium, nickel from cobalt and zinc from cadmium. The zirconium-hafnium thiocyanate system has been in use for decades in the production of reactor-grade zirconium and hafnium metals. The other systems are being used in analytical chemistry and they do have the potential for commercial-scale applications.
Other sources of HCN formation via the reaction between light or atmospheric oxygen and thiocyanic acid would not be significant due to the slow reaction rate under normal conditions.
Published kinetic data show that temperature, thiocyanic acid concentration and total acidity promote all the reactions listed above. Thus, environmental, thiocyanate consumption (economics) and solids buildup problems can all be minimized by practicing the metal separation processes at relatively low temperature, low thiocyanic acid concentration and low total acidity.