Table of Contents
Almost all aluminum in the United States is produced from imported bauxite or alumina. This country has, however, more than adequate domestic nonbauxitic alumina resources that could alleviate dependency on foreign sources. For both economic and security reasons, the Federal Government has had a longstanding interest in the development of alternative technology that would allow utilization of those resources.
In 1927, the Bureau of Mines published a comparative study on extracting aluminum from clay with sulfuric, nitric, and hydrochloric acids. Alumina research was resumed during World War II, when the Defense Plant Corporation funded four pilot plant studies and the National Bureau of Standards, under the sponsorship of the Army Signal Corps, investigated an HCl process for extracting alumina from kaolinitic clay.
In 1973, a program was Initiated by the Bureau of Mines to evaluate the technical and economic potential of several processes for producing alumina from domestic resources. An HCl-clay process was judged the best application of existing technology. At the same time, bench-scale investigations were conducted on alternative technologies which might avoid some of the disadvantages of the HCl processes.
A key operation in the HCl-clay process is the thermal decomposition of aluminum chloride hexahydrate (ACH) to the final alumina product. This step is energy intensive, and the viability of the process would be improved if the energy requirement for decomposition was decreased. One possible way to achieve the decrease is to modify the process so that its aqueous operations did not produce ACH, but a basic chloride with a composition between ACH and alumina.
In the HCl-clay process, kaolin is calcined to an amorphous mixture of alumina and silica. The calcined kaolin is leached with HCl solution, and aluminum is dissolved according to the following reaction:
Al2O3 + 6HCl = 2AlCl3 + 3H2O……………………………………………….(1)
The AlCl3 solution is evaporated or sparged with HCl gas, and the solid, AlCl3·6H2O, is formed.
During bench-scale research on HCl leaching of calcined clay, sometimes the apparent extraction of Al2O3, was greater than 100 pct. Examination of the data indicated that aluminum could be dissolved from kaolin with solutions containing less HCl than required by reaction 1. The resulting solutions contained basic aluminum chloride.
A literature search revealed reports on the solubility of Al(OH)3 and Al2O3 in solutions of AlCl3. A study by Breuil defined the compositions of several crystalline basic aluminum chlorides obtained by extended aging of mixtures of Al(OH)3, AlCl3, and H2O at different temperatures.
The formation of the basic chlorides of aluminum may be represented by either of the following reactions;
(½)Al2O3 + YHCl = Al(OH)xCly + (3/2-X)H2O…………………………………………..(2)
(X/6)Al2O3 + (Y/3)AlCl3 + (X/2)H2O = Al(OH)xCly……………………………………(3)
where X + Y = 3. Reaction 2 is a general expression for reaction 1 and leads to the basic chloride when reactive Al2O3 is in excess. When X is greater than 3/2, the coefficient for H2O becomes negative and water is consumed as the reaction proceeds to the right. Equation 3 expresses the direct reaction of AlCl3 with Al2O3, which also consumes water. The value of Y is the molar ratio of Cl:Al. This ratio will be used as an indicator of composition, For example, Cl:Al for ACH is three, for alumina it is zero, and the basic chlorides are intermediate.
The objectives of this study were to determine the best Cl:Al ratio for extracting aluminum from calcined kaolin, and to determine what, if any, basic aluminum chloride solids could be recovered from the pregnant solution.
Materials Equipment and Procedures
Raw clay was obtained from the Theile Kaolin Co., Sanderville, GA, and is representative of very large deposits of eastern Georgia kaolinitic clay. The clay was dried for 48 h at 100° C, ground with a disk pulverizer to minus 60 mesh, and blended. Analysis by a combination of wet-chemical methods and inductively coupled plasma emission spectroscopy gave the results shown in table 1.
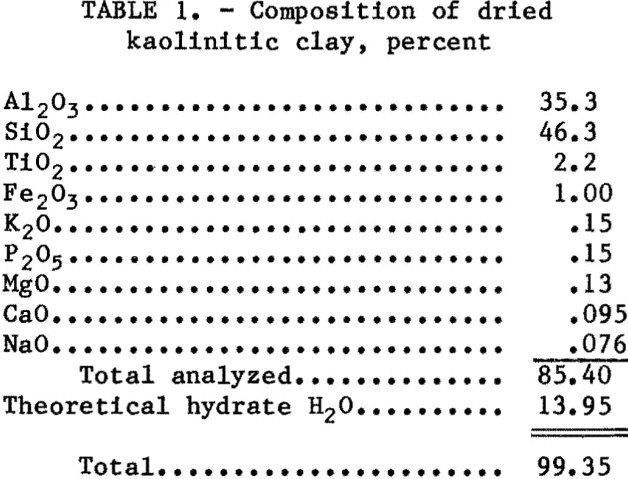
The dried clay was calcined prior to leaching. The calcined clay contained 41.1 pct Al2O3. In tests to determine the effects of calcination time and temperature, a 70- to 100-g sample of clay in an uncovered crucible was placed in a preheated electric furnace. After the specified time had elapsed, the crucible was removed and allowed to air-cool. For larger samples the clay was roasted for 4 to 8 h.
Leaching tests, both single-stage and countercurrent, were made in a 0.5-or 1-L glass resin kettle heated with an electric mantle and vented through a water-cooled condenser. The calcined clay charge and leaching solution were placed in the kettle, heated to boiling, and stirred for the specified time. The test was terminated by vacuum filtration of the hot contents of the kettle on a Buchner funnel. The filter cake was washed with three 100 mL portions of water acidified to pH 3 with HCl. The combined filtrate and wash were evaporated to concentrate the contained aluminum salts. At the first sign of crystallization, the liquor was allowed to cool at room temperature overnight. The crystals were filtered from the liquor, washed by slurrying in propanol, refiltered, and air-dried. The leach residue, combined filtrate, and crystals were analyzed for Al, Cl, and the major impurities Fe, Mg, P, Ca, Na, and K.
Reagent-grade HCl and ACH were used in the tests.
Results and Discussion
Effects of Calcination Time and Temperature
The effects of thermal treatment on the acid solubility of kaolinite are well established. The natural mineral is resistant to acid attack. On heating to 400° C, kaolinite exhibits little change other than minor losses of free moisture. Between 425° and 525° C and depending on the degree of crystallinity, the kaolinite structure collapses and loses hydrate water amounting to a theoretical 13.95 pct. The resultant material is almost amorphous to X-ray diffraction, and its alumina content is almost totally soluble in acids. On further heating, no detectable changes occur until about 900° C when mullite, gamma alumina, and crystobalite begin to form. At this point, the acid solubility decreases abruptly.
The solubility window between calcination temperatures of 500° and 850° C is illustrated in figure 1. The lower curve shows the weight loss for dry kaolin after heating to the temperatures indicated. The upper curve is the percent of aluminum extracted by leaching with 26- pct HCl. The same type of behavior was expected when initial experiments were conducted with an AlCl3 leachant for kaolin calcined at temperatures within the window. Poor reproducibility of extraction results led to a controlled calcination study. The results of that study are also shown in figure 1.
The calcination temperature was critical for extraction of aluminum from kaolin with a 30-pct solution of AlCl3. Rather than a plateau region of solubility, extraction with AlCl3 increased with temperature until a sharp decrease occurred at more than 850° C.
The effect of calcination time at 850° C on extraction was studied over the range 0.25 to 9 h. Extraction of aluminum was determined for a 30-pct AlCl3 solution and attained a constant level for kaolin calcined for more than 1 h. No decrease in extractibillty caused by overroasting was observed for samples calcined for 9 h. All subsequent leaching tests were made with clay calcined at 850° C for 4 to 8 h.
Single-Stage Leaching and Crystallization
Three series of leaching tests were made with 25- to 200-g charges of calcined clay and 72 g AlCl3 as a 10-, 20-, or 30-pct solution. The amount of leachant for each series was 720, 360, or 240 g, and the initial Cl:Al ratio for the total material in the leaching reactor for each test ranged from 2.18 to 0.75 within a series. All test solutions were maintained at boiling and stirred for 2 h. Results of these tests are shown in figure 2. Aluminum extraction ranged from about 57 to 87 pct and increased with increasing concentration of AlCl3 in the leachant at constant initial Cl:Al. The extraction decreased when the initial Cl:Al was decreased for each AlCl3 concentration. Aluminum extraction is maximized by leaching with a high initial Cl:Al ratio and high concentration of chloride.
The lower line in figure 2 shows the relationship between the Cl:Al of the pregnant liquor and the initial ratio. The liquor ratios obtained were parallel to and about 0.2 unit higher than the initial Cl:Al. The liquor ratio was not influenced by the AlCl3 concentration of the leachant.
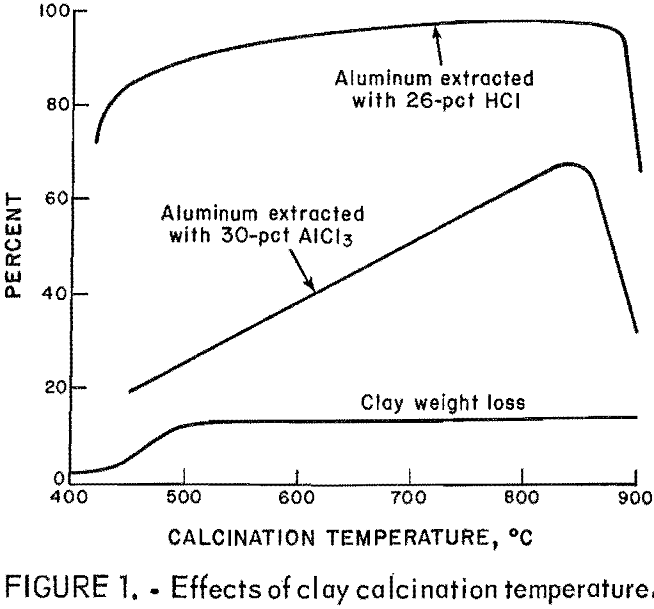
In tests with initial Cl:Al ratios less than about 1.5, difficulties were encountered because of the high initial solids content. The problem became worse as the test progressed and water was consumed by reaction 3. Filtration and washing of the larger amounts of leach residue also were difficult.
The time required for dissolution was determined in a series of tests with 30- pct AlCl3 and initial Cl:Al of 1.8. With test durations from 0.5 to 16 h, aluminum extraction was 83 pct in 1 h and did not increase by more than the estimated experimental error of 3 pct in 16 h.
The compositions of the solids produced by evaporation and cooling of single-stage pregnant liquors were poorly reproducible, with Cl:Al ratios ranging from 3.0 to 1.4 and correlated approximately with the liquor ratio. No solid products were obtained from tests with initial ratios below 1.4 because the pulp density problem was compounded by increasingly viscous pregnant liquors, which tended to supersaturate.
The best conditions for single-stage leaching are shown in table 2. Using a 30-pct AlCl3 leachant and an initial Cl:Al ratio of 2.2 gave the best trade-off between high aluminum extraction and low liquor Cl:Al ratio. The liquors were reasonably easy to filter and gave basic chloride crystals on evaporation.
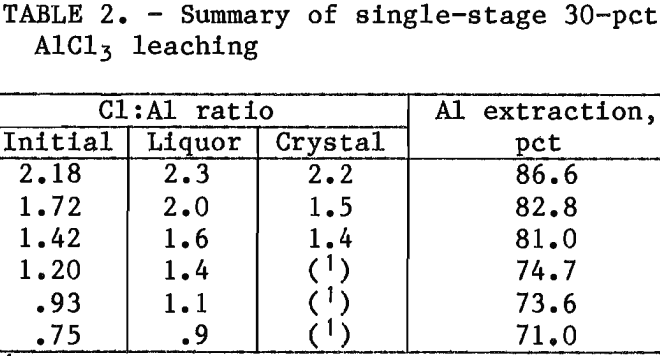
An important result of single-stage leaching is the concentration of aluminum in the pregnant liquor. The 30-pct AlCl3 leachant in the tests summarized in table 2 was initially just below its saturation concentration of 31 pct, which is equivalent to an aluminum concentration of 6.3 pct. The pregnant liquors from the single-stage tests were not analyzed for aluminum before being diluted with wash water, but from the amounts of alumina dissolved, the aluminum concentration of the liquor was significantly higher than 6.3 pct. Since aluminum loading of HCl- clay process liquors is limited to saturated AlCl3, the higher aluminum concentration possible with basic chloride solutions has the important potential benefit of decreasing the size of the processing equipment.
Countercurrent Leaching and Crystallization
Countercurrent leaching was studied as a means of avoiding the trade-off that was necessary in single-stage leaching between high alumina extraction and low Cl:Al ratios. The countercurrent method should also avoid high pulp densities and the associated handling problems. A three-stage leaching operation was chosen.
The general countercurrent leaching scheme is shown in figure 3. Calcined clay enters the array from the left and moves to the right. The leachant moves from top to bottom. Nine batch tests are required to generate the intermediate compositions needed to duplicate a
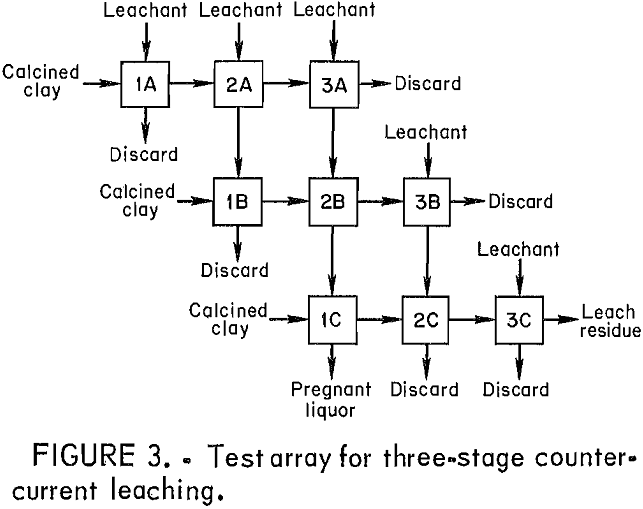
continuous three-stage countercurrent leaching operation. The liquid stream of importance enters as leachant for step 3A and exits from step 1C. This liquor has been contacted three times with increas-ingly alumina-rich ore and represents the pregnant liquor from countercurrent leaching. The important solid stream enters at 1C and exits at 3C, where it represents the final leach residue.
The composition and amount of leachant and the amount of calcined clay were chosen so that the overall Cl:Al ratio would be below 1.5, an area that was very troublesome in the single-stage tests. To provide sufficient water for the reaction while maintaining the chloride concentration, the leachant of choice was not an AlCl3 solution, but HCl. With the proper balance of leachant to clay, all of the HCl would be consumed in the first leaching stage and produce an AlCl3 leachant for the second stage. All of the aluminum in the final pregnant liquor was derived from the clay.
The leachant chosen was 110 g (103 mL) of 15-pct HCl, and three test arrays were run with calcined clay charges of 40, 50, and 60 g, which corresponded to initial Cl:Al ratios of approximately 1.4, 1.1, and 0.9. Each leaching test was run for 2 h, and the slurry was filtered without washing. The residue was passed to the next test to the right and the filtrate to the next lower test. Only the final residue from test 3C was washed before analysis to determine aluminum extraction.
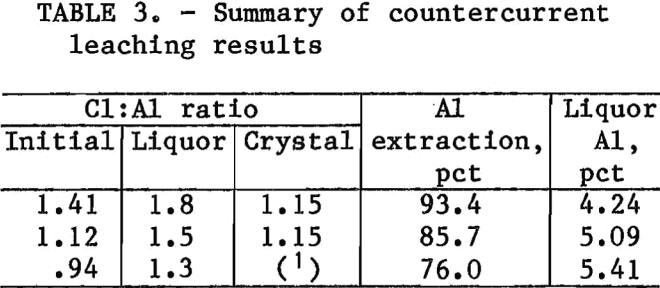
Table 3 summarizes the results of countercurrent leaching tests. The 93.4-pct aluminum extraction for the test with an initial Cl:Al ratio of 1.41 is significantly better than was possible in single-stage leaching and is comparable to the 95 pet achieved in the HCl-clay process. Liquor Cl:Al ratios for all three tests were about 0.4 unit higher than the initial ratio. Few problems were encountered in filtering liquors from leach residues except for the test with an initial Cl:Al ratio of 0.94. That liquor was very viscous, and filtration was difficult. Concentrations of aluminum in pregnant liquors ranged from 4.2 to 5.4 pct.
The solids obtained on evaporation and cooling of liquors with Cl:Al ratios of 1.8 and 1.5 were crystalline and fine and had a Cl:Al ratio of 1.15. An X-ray diffraction pattern was obtained and matched the pattern given by Breuil for a compound which she identified as 5AlCl3·8Al(OH)3·37.5H2O. No solid could be obtained from the liquor with a Cl:Al ratio of 1.30. Evaporation only increased its viscosity and gave a syrup reminiscent of a supersaturated organic solution or a concentrated colloidal suspension.
Although Breuil’s preparation of the above compound, which is herein designated ACHH, involved aging for a long time, research by the authors determined that it can be prepared reproducibly from basic aluminum chloride solutions with Cl:Al ratios in the range of 2.0 to 1.4 by evaporation to incipient crystallization and cooling. From solutions at the upper end of the range, the solid obtained is a mixture of ACH and ACHH. At the low end, syrup formation is a problem. A solution with a ratio of 1.8 is near ideal for single-phase ACHH. The pure ACHH prepared for thermal decomposition studies was crystallized from a solution with a Cl:Al ratio of 1.6 and was made by dissolving aluminum shot in HCl.
Purification and Solubility Studies
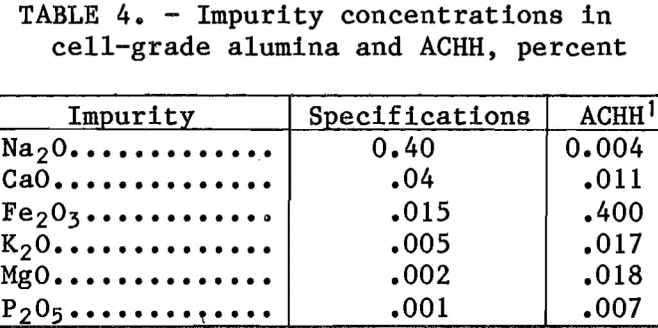
The levels of major impurities in the ACHH obtained from countercurrent leaching of clay with a liquor Cl:Al ratio of 1.5 are given in table 4.
Concentrations are expressed as percent of impurity oxide in alumina derived from the ACHH. Specifications for cell-grade alumina are given for comparison. These specifications were developed by a sub-committee of the Industry/Bureau of Mines Steering Committee for the Alumina Mini-plant Program. Considerable improvement is necessary if a cell-grade product is to be produced from ACHH. A cursory examination was made of three possible methods for improving the purity of the ACHH.
The crystal washing procedure with propanol was adopted for experimental expedience. Purification by redissolving the propanol-washed ACHH in water, recrystallizing, and washing a second time with propanol was attempted. Some purification was noted, but ACHH was significantly soluble in propanol. Countercurrent washing with an alcohol saturated with ACHH may be possible but was not investigated.
Attempts were made to remove the iron from pregnant liquors by solvent extraction with amine extractants, but were unsuccessful because the liquors did not contain sufficient free chloride to form the chlorocomplexes of iron that the amines could extract.
A third purification approach was partially successful. A pregnant liquor was subjected to electrolytic reduction to reduce the contained iron to the ferrous state. The liquor was then evaporated and crystallized. The reason for reducing the iron to the ferrous state was to retard iron substitution for aluminum in the ACHH crystal lattice. Decreasing the amount of ferric iron substitution should also allow more perfect crystal growth and decreased inclusion of other impurities. The level of iron in ACHH was decreased by two-thirds with this method, and minor decreases of other impurities were noted. Problems, including air leaks into the equipment, prevented maintaining more than 90 to 95 pct of the dissolved iron in the ferrous state during crystallization.
The greater concentration of aluminum in the basic chloride liquors compared with that in HCl-clay liquor is an important processing advantage. The leaching tests were not optimized for high liquor concentration. To determine the maximum concentration of aluminum achievable, a series of synthetic liquors with Cl:Al ratios ranging from 2.4 to 1.2 were prepared, evaporated until crystallization began, and stored in tightly capped bottles at room temperature for 4 to 6 wk, after which the solids and supernatant liquids were analyzed. The solid phase in contact with the higher ratio liquors was a mixture of ACHH and ACH; with the lower ratio liquors, another basic chloride, with a diffraction pattern identified by Breuil as AlCl3·4Al(OH)3·7.5H2O, was formed. In the middle-range liquor ratios of 2.0 to 1.5, the solids were single-phase ACHH, and the aluminum concentration of the supernatant was 9.3 to 10.9 pct.
Thermal Decomposition of ACHH
A sample of pure ACHH prepared as previously described was submitted to the Thermodynamics Group of the Bureau’s Albany Research Center. Comparative determinations were made by scanning differential calorimetry of the energy requirements for decomposition of both ACHH and ACH to gamma alumina. The results showed that ACHH requires only 61.6 pct as much energy for decomposition as ACH. This a very significant decrease because approximately one-half of the total energy requirement of the HCl-clay process is consumed in the decomposition step.
Discussion and Conclusions
The following major conclusions have resulted from this study;
- About 90 pct of the alumina content of calcined kaolin clay can be leached with a solution of AlCl3 or substoichiometric HCl to give a basic chloride liquor.
- Calcination temperature is critical. About 850° C is the best for clay to be leached by the basic chloride method. This is in contrast to standard HCl leaching, where a broad range of calcination temperatures may be used.
- The potential aluminum concentration in basic chloride pregnant liquor is about 75 pct higher than is possible in liquor from standard HCl leaching.
- The basic chloride liquor from countercurrent leaching can be crystallized to give the compound 5AlCl3·8Al(OH)3·37·5H2O.
- The energy needed to decompose ACHH to alumina is about 62 pct of that needed for the ACH produced in the HCl-clay process.
This research should be considered a preliminary effort toward the possible development of a new process for extracting aluminum from clays. Much more research is required before a preliminary flowsheet could be proposed. While countercurrent leaching is probably the best approach for this system, detailed optimization studies are needed to balance extraction with liquor aluminum concentration, Cl:Al ratio, and ease of filtration and crystallization.
